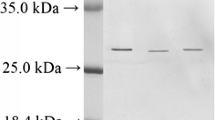
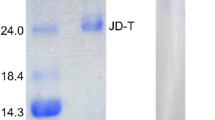
Summary
The trypsin of three species of frogs (Rana esculenta, R. ridibunda, R. temporaria), of the tadpoles ofR. temporaria, of two species of fish (Salmo trutta, Tinca tinca) and of the lizard,Lacerta muralis, were separated by agar gel electrophoresis, eluted and partly characterized.
-
1.
Three isozymes were found in the frogs, one in the tadpole, three in the trout, four in the tench and two in the lizard. The isozyme pattern of the frogs is remarkably constant whereas those of the fishes and the lizard appear to be more variable under the influence of environmental factors. The single trypsin of the tadpole does not resemble any of the adult trypsins ofR. temporaria.
-
2.
The affinity for DL-BAPA of all isozymes of trypsin examined varies by a factor of approximately 20 (Table 1), but in most cases there is little dependence ofK m (app) on temperature and pH in the physiological range.
-
3.
In amphibians and reptiles substrate affinity (expressed asK m (app)) and stability (expressed as the half life at pH 8.2 and 30° C) are negatively correlated, whereas the isozymes of fishes (with the exception of T2 of the tench), despite their relatively high affinity, are stable at a pH of 8.2.
Similar content being viewed by others
References
Baldwin, J., Hochachka, P. W.: Functional significance of isoenzymes in thermal acclimation: acetylcholinesterase from trout brain. Biochem. J.116, 883–887 (1970)
Barnard, E. A., Hope, W. C.: Identification of histidine in the active center of chymotrypsins from a reptile and a fish. Biochim. biophys. Acta (Amst.)178, 364–369 (1969)
Barrington, E. J. W.: Gastric digestion in the lower vertebrates. Biol. Rev.17, 1–26 (1942)
Blacher, L. J., Liosner, L. D.: Untersuchungen über Mechanik der Funktionsgenese bei der Amphibienmetamorphose. II. Veränderungen der proteolytischen Funktion des Darmes im Prozeß der Metamorphose bei Rana temporaria. Biol. Zbl.50, 285–292 (1930)
Bradshaw, R. A., Neurath, H., Tye, R. W., Walsh, K. A., Winter, W. P.: Comparison of the partial amino-acid sequence of dogfish trypsinogen with bovine trypsinogen. Nature (Lond.)226, 237–239 (1970)
Coan, M. H., Travis, J.: Comparative biochemistry of proteases from a coelenterate. Comp. Biochem. Physiol.32, 127–139 (1970)
Croston, C. B.: Endopeptidases of salmon ceca: chromatographic separation and some properties. Arch. Biochem. Biophys.112, 218–223 (1965)
Gates, B. J., Travis, J.: Isolation and comperative properties of shrimp trypsin. Biochemistry8, 4483–4489 (1969)
Hofer, R.: Einfluß von Temperatur, Photoperiode und Jahreszeiten auf Verdauung und Atmung zweier Froscharten: Rana ridibunda (bzw. Rana esculenta) and Rana temporaria. Zool. Jb. Abt. Physiol.76, 507–530 (1972)
Hofer, R., Ladurner, H., Gattringer, A., Wieser, W.: Relationship between the temperature preferenda of fishes, amphibians and reptiles, and the substrate affinities of their trypsins. J. comp. Physiol.99, 345–355 (1975)
Jany, K. D.: Isolierung und Charakterisierung von Serin-Endopeptidasen des magenlosen Fisches Carassius auratus gibelio. Diss., Univ. Heidelberg (1972)
Möckel, W., Barnard, E. A.: Isolation and properties of some reptilian and fish chymotrypsins. Biochim. biophys. Acta (Amst.)178, 354–363 (1969)
Nilsson, A., Fänge, R.: Digestive proteases in the holocephalian fish Chimaera monstrosa. Comp. Biochem. Physiol.31, 147–165 (1969)
Pfleiderer, G., Zwilling, R.: Die molekulare Evolution proteolytischer Enzyme. Naturwissenschaften59, 396–405 (1972)
Prahl, J. W., Neurath, H.: Pancreatic enzymes of the spiny Pacific dogfish. I. Cationic chymotrypsinogen and chymotrypsin. Biochemistry5, 2131–2146 (1966)
Reeck, G. R., Winter, W. P., Neurath, H.: Pancreatic enzymes of the African lungfish Protopterus aethiopicus. Biochemistry9, 1398–1403 (1970)
Reeder, W. G.: The digestive system. In: Physiology of the amphibia (ed. Moore, J. A.), p. 99–149. New York and London: Academic Press 1964
Travis, J., Liener, I. E.: The crystallization and partial characterization of porcine trypsin. J. biol. Chem.240, 1962–1966 (1965)
Tuppy, H., Wiesbauer, U., Wintersberger, E.: Aminosäure-p-nitroanilid als Substrat für Aminopeptidasen und andere proteolytische Fermente. Hoppe-Seylers Z. physiol. Chem.329, 278–288 (1962)
Walsh, K. A.: Trypsinogens and trypsins of various species. In: Methods in enzymology-XIX: Proteolytic enzymes, Perlmann, G. E. and Lorand, L., (eds.), p. 41–63. New York and London: Academic Press 1970
Walsh, K. A., Neurath, H.: Trypsinogen and chymotrypsinogen as homologous proteins. Proc. nat. Acad. Sci. (Wash.)52, 884–899 (1964)
Zwilling, R., Pfleiderer, G., Sonneborn, H. H., Kraft, V., Stucky, L.: The evolution of endopeptidases V. Common and different traits of bovine and crayfish trypsin. Comp. Biochem. Physiol.28, 1275–1287 (1969)
Zwilling, R., Tomasek, V.: Amino-acid composition of crayfish trypsin. Nature (Lond.)228, 57–58 (1970)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hofer, R., Schiestl, W., Gattringer, A. et al. Trypsin isozymes of some ectothermic vertebrates. J Comp Physiol B 101, 111–119 (1975). https://doi.org/10.1007/BF00694152
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00694152