Summary
-
1.
Oxygen consumption and lactate content of the lizardDipsosaurus dorsalis were determined under standard conditions and for a bout of maximal activity induced by a 2-min period of electrical stimulation. Observations were made between 25 ° and 45 °C.
-
2.
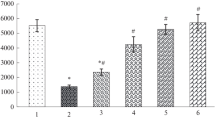
Maximal aerobic scope, 2.27 cm3 O2/(g × hr), occurred at 40 °C (Figs. 2, 4). The increase in oxygen consumption during activity at the various temperatures between 25 ° and 45 °C represented 7- to 17-fold of corresponding resting levels.
-
3.
Lactate content of restingDipsosaurus is independent of temperature and averages 0.25 mg/g body weight. Maximal lactate production during the activity induced by a 2-min period of electrical stimulation occurred at 40 °C (Fig. 3). The capacity ofDipsosaurus for anaerobic metabolism exceeds that of other lizards investigated, both in its magnitude and in its thermal dependence.
-
4.
The total amount of energy mobilized byDipsosaurus in the activity induced by a 2-min period of electrical stimulation was maximal at 40 °C (Fig. 4). Anaerobiosis accounts for a minimum of 58–83% of the total energetic expenditure.
-
5.
It is postulated that the principal physiological adaptations to preferred thermal levels in reptiles have involved energy mobilization during and rapid recovery after activity.
Similar content being viewed by others
References
Asplund, K. K.: Metabolic scope and body temperatures of whiptail lizards (Cnemidophorus). Herpetologica26, 403–411 (1970).
Beckman, W. A., Mitchell, J. W., Porter, W. P.: Thermal model for prediction of a desert iguana's daily and seasonal behavior. Amer. Soc. Mech. Engineers Publ. 71-WA/HT-35, 7 (1971).
Belkin, D. A.: The running speeds of the lizardsDipsosaurus dorsalis andCallisaurus draconoides. Copeia1961, 223–224 (1961).
Bennett, A. F.: Oxygen transport and energy metabolism in two species of lizards,Sauromalus hispidus andVaranus gouldii. Ph. D. Thesis. Ann Arbor: Univ. of Michigan 1971.
Bennett, A. F.: A comparison of activities of metabolic enzymes in lizards and rats. Comp. Biochem. Physiol.42B, 637–647 (1972a).
Bennett, A. F.: The effect of activity on oxygen consumption, oxygen debt, and heart rate in the lizardsVaranus gouldii andSauromalus hispidus. J. Comp. Physiol.79, 259–280 (1972b).
Bennett, A. F., Dawson, W. R.: Reptilian metabolism. In: Biology of the Reptilia. Physiology A, vol. 5, ed. C. Gans. New York: Academic Press (in press).
Bennett, A. F., Licht, P.: Anaerobic metabolism during activity in lizards. J. Comp. Physiol.81, 277–288 (1972).
Cook, S. F.: Respiratory metabolism of certain reptiles and amphibia. Univ. Calif. Publ. Zool.53, 367–376 (1949).
Dawson, W. R., Bartholomew, G. A.: Relation of oxygen consumption to body weight, temperature, and temperature acclimation in lizardsUta stansburiana andSceloporus occidentalis. Physiol. Zool.29, 40–51 (1956).
Dawson, W. R., Bartholomew, G. A.: Metabolic and cardiac responses to temperature in the lizardDipsosaurus dorsalis. Physiol. Zool.31, 100–111 (1958).
Dawson, W. R., Poulson, T. L.: Oxygen capacity of lizard bloods. Amer. Midl. Nat.68, 154–164 (1962).
Dawson, W. R., Templeton, J. R.: Physiological responses to temperature in the lizardCrotaphytus collaris. Physiol. Zool.36, 219–236 (1963).
DeWitt, C. B.: Behavioral thermoregulation in the iguanid lizard,Dipsosaurus dorsalis. Ph. D. Thesis. Ann Arbor: Univ. of Michigan 1963.
DeWitt, C. B.: Precision of thermoregulation and its relation to environmental factors in the desert iguana,Dipsosaurus dorsalis. Physiol. Zool.40, 49–66 (1967a).
DeWitt, C. B.: Behavioral thermoregulation in the desert iguana. Science158, 809–810 (1967b).
Dill, D. B.: Life, heat, and altitude. Physiological effects of hot climates and great heights. Cambridge: Harvard Univ. Press 1938.
Dill, D. B., Edwards, H. T., Book, A. V., Talbott, J. H.: Properties of reptilian blood. III. The chuckwalla (Sauromalus obesus Baird). J. cell. Comp. Physiol.6, 37–42 (1935).
Fry, F. E. J.: Effects of the environment on animal activity. Pub. Ont. Fish. Res. Lab. No68, 1–62 (1947).
Hemmingsen, A. M.: Energy metabolism as related to body size and respiratory surfaces, and its evolution. Rep. Steno Mem. Hosp. Nord. Insulinlab.9, 1–110 (1960).
Hill, R. W.: Determination of oxygen consumption using the paramagnetic oxygen analyzer. J. appl. Physiol.33, 261–263 (1972).
Licht, P.: Effects of temperature on heart rates of lizards during rest and activity. Physiol. Zool.38, 129–137 (1965).
Mayhew, W. W.: Growth response to photoperiodic stimulation in the lizardDipsosaurus dorsalis. Comp. Biochem. Physiol.14, 209–216 (1965a).
Mayhew, W. W.: Hibernation in the horned lizard,Phrynosoma m'calli. Comp. Biochem. Physiol.16, 103–119 (1965b).
Mayhew, W. W.: Biology of desert amphibians and reptiles, p. 195–356. In: Desert biology, ed. G. W. Brown, Jr. New York: Academic Press 1968.
Mayhew, W. W.: Reproduction in the desert lizard,Dipsosaurus dorsalis. Herpetologica27, 57–77 (1971).
Minnich, J. E.: Water and electrolyte balance of the desert iguana,Dipsosaurus dorsalis, in its natural habitat. Comp. Biochem. Physiol.35, 921–933 (1970a).
Minnich, J. E.: Evaporative water loss from the desert iguana,Dipsosaurus dorsalis. Copeia1970, 575–578 (1970b).
Minnich, J. E., Shoemaker, V. H.: Diet, behavior and water turnover in the desert iguana,Dipsosaurus dorsalis. Amer. Midl. Nat.84, 496–509 (1970).
Moberly, W. R.: Hibernation in the desert iguana,Dipsosaurus dorsalis. Physiol. Zool.36, 152–160 (1963).
Moberly, W. R.: The metabolic responses of the common iguana,Iguana iguana, to activity under restraint. Comp. Biochem. Physiol.27, 1–20 (1968a).
Moberly, W. R.: The metabolic responses of the common iguana,Iguana iguana, to walking and diving. Comp. Biochem. Physiol.27, 21–32 (1968b).
Norris, K. S.: The ecology of the desert iguanaDipsosaurus dorsalis. Ecology34, 265–287 (1953).
Pough, F. H.: Environmental adaptations in the blood of lizards. Comp. Biochem. Physiol.31, 885–901 (1969).
Privitera, C. A., Mersmann, H. J.: Seasonal oxidative phosphorylation by turtle heart mitochondria. Comp. Biochem. Physiol.17, 1045–1048 (1966).
Roberts, L. A.: Oxygen consumption in the lizardUta stansburiana. Ecology49, 809–819 (1968).
Shoemaker, V. H., Licht, P., Dawson, W. R.: Thermal dependence of water and electrolyte excretion in two species of lizards. Comp. Biochem. Physiol.23, 255–262 (1967).
Weathers, W. W.: Physiological thermoregulation in the lizardDipsosaurus dorsalis. Copeia1970, 549–557 (1970).
Wilson, K. J.: The relationships of activity, energy, metabolism, and body temperature in four species of lizards. Ph. D. Thesis. Clayton, Victoria: Monash Univ. 1971.
Author information
Authors and Affiliations
Additional information
This study was supported in part by a Miller Postdoctoral Research Fellowship to AFB and by National Science Foundation Grant GB-25022 to WRD. We thank Dr. Lon McClanahan for assistance in collecting the animals.
Rights and permissions
About this article
Cite this article
Bennett, A.F., Dawson, W.R. Aerobic and anaerobic metabolism during activity in the lizardDipsosaurus dorsalis . J. Comp. Physiol. 81, 289–299 (1972). https://doi.org/10.1007/BF00693633
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00693633