Abstract
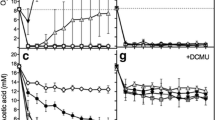
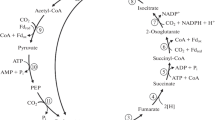
Rhizobium ORS 571, isolated from stem nodules of the tropical legumeSesbania rostrata is able to grow in the chemostat with molecular nitrogen as sole nitrogen source at a specific growth rate of 0.1 h-1. Samples from nitrogenfixing cultures showed high acetylene reduction activities: 1,500 nmol ethylene formed per milligram dry weight per hour. Under nitrogen-fixing conditions an uptake hydrogenase is induced. Ammonia-assimilating cultures, without additional hydrogen, did not induce hydrogenase. The addition of hydrogen to succinate-limited nitrogen-fixing cultures resulted in an increase in the molar growth yield on succinate (Y succinate) from 27 to 35 and a slight decrease in the molar growth yield on oxygen (\(Y_{O_2 }\)), showing that hydrogen oxidation is less energy-yielding than the oxidation of endogenous substrates. Respiration-driven proton translocation measured with starved cells indicated the functioning of site 1 and 2 of oxidative phosphorylation. Cytochrome spectra showed that cytochromea 600, present at high dissolved oxygen tension (d.o.t.) almost completely disappeared at low d.o.t. In flash-photolysis spectra only thea-type cytochrome could be detected as an oxidase in cells both grown at high and low d.o.t. Growth yields in ammonia-assimilating cultures were higher than those measured in nitrogen-fixing cultures. Assuming two sites of oxidative phosphorylation, a molar growth yield on ATP (Y ATP) of about 3 and 6 was calculated for respecticely nitrogen-fixing and ammonia-assimilating cultures. TheY ATP under nitrogen-fixing conditions is dependent on the amount of H2 formed per mol N2 fixed (H2/N2 ratio). A method has been described to calculate the total amount of ATP use by nitrogenase during the fixation of 1 mol N2 (ATP/N2 ratio) and H2/N2 ratios in aerobic nitrogen fixing organisms. This calculation yielded that nitrogen fixation inRhizobium ORS 571 is a high ATP-consuming process. The calculated ATP/N2 and H2/N2 ratios were respectively 42 and 7.5.
Similar content being viewed by others
Abbreviations
- d.o.t.:
-
dissolved oxygen tension
References
Agarwal AK, Keister DL (1983) Physiology of explanta nitrogenase activity inRhizobium japonicum. Appl Environ Microb 45:1592–1601
Albrecht SL, Maier RJ, Hanus FJ, Russell SA, Emerich DW, Evans HJ (1979) Hydrogenase inRhizobium japonicum increases nitrogen fixation by nodulated soybean. Science 203:1255–1257
Appleby CA (1969a) Electron transport systems ofRhizobium japonicum. I. Haemoproteins P450, other CO reactive pigments, cytochromes and oxidases in bacteroids from N2-fixing root nodules. Biochim Biophys Acta 172:71–87
Appleby CA (1969b) Electron transport system ofRhizobium japonicum. II.Rhizobium haemoglobin, cytochromes and oxidases in free-living cultured cells. Biochim Biophys Acta 172:88–105
Boogerd FC, Verseveld HW van, Stouthamer AH (1981) Respiration driven proton translocation with nitrite and nitrous oxide inParacoccus denitrificans. Biochim Biophys Acta 638:181–191
Boogerd FC, Verseveld HW van, Stouthamer AH (1983) Dissimilatory nitrate uptake inParacoccus denitrificans via a δμH+ dependent system and a nitrate-nitrite antiport system. Biochim Biophys Acta 723:415–427
Bowien B, Schlegel HG (1981) Physiology and biochemistry of aerobic hydrogen-oxidizing bacteria. Ann Rev Microbiol 35:405–452
Carter KR, Jennings NT, Hanns J, Evans HJ (1978) Hydrogen evolution and uptake by nodules of soy beans inoculated with different strains ofRhizobium japonicum. Can J Microbiol 24:307–311
Cypionka H, Meyer O (1982) Influence of carbon monoxide on growth and respiration of carboxydobacteria and other aerobic organisms. FEMS Microbiol Lett 15:209–214
Daesch G, Mortensen LE (1967) Sucrose catabolism inClostridium pasteurianum and its relation to N2 fixation. J Bacteriol 96:346–351
Dalton H, Postgate JR (1969) Growth and physiology ofAzotobacter chroococcum in continuous culture. J Gen Microbiol 56:307–319
Dixon ROD (1972) Hydrogenase in legume root nodules bacteroids: occurrence and properties. Arch Microbiol 85:193–201
Dixon ROD, Blunder EAG, Searl JW (1981) Intracellular space and hydrogen diffussion in pea and lupin root nodules. Plant Sci Lett 23:109–116
Dreyfus BL, Dommergues YR (1981) Nitrogen fixing nodules induced byRhizobium on the stem of the tropical legumeSesbania rostrata. FEMS Microbiol Lett 5:369–372
Eisbrenner G, Evans HJ (1982) Carriers in electron transport from molecular hydrogen to oxygen inRhizobium japonicum bacteroids. J Bacteriol 149:1005–1012
Eisbrenner G, Hickok RE, Evans HI (1982) Cytochrome patterns inRhizobium japonicum cells grown under chemolithotrophic conditions. Arch Microbiol 132:230–235
Elmerich C, Dreyfus BL, Reijsset, G, Aubert IP (1982) Genetic analysis of nitrogen fixation in a tropical fast-growingRhizobium. EMBO J 4:499–503
Elmerich C, Dreyfus BL, Aubert JP (1983) Nicotinic acid requirement and degradation bySesbania rhizobium strain ORS 571. FEMS Microbiol Lett 19:281–284
Emerich DW, Ruiz-Argueso T, Ching TM, Evans HI (1979) Hydrogen dependent nitrogenase activity and ATP formation inRhizobium japonicum bacteroids. J Bacteriol 137:153–160
Hill S (1976) The apparent ATP requirement for nitrogen fixation in growingKlebsiella pneumoniae. J Gen Microbiol 95:297
Hollander H de, Stouthamer AH (1980) The electron transport chain ofRhizobium trifolii. Eur J Biochem 111:473–478
Hollander H de (1981) Studies on the physiology ofRhizobium trifolii. Ph.D. thesis, Vrije Universiteit, Amsterdam
Hüdig H, Drews G (1982) Characterization of ab type cytochromec oxidase ofRhodopseudomonas capsulata. FEBS Lett 146:389–393
Jones RW (1980) The role of the membrane bound hydrogenase in the energy conserving oxidation of molecular hydrogen byEscherichia coli. Biochim J 188:345–350
Kretovich WL, Romanov VI, Korolyov AV (1973)Rhizobium leguminosarum cytochromes (Vicia faba). Plant Soil 39:619–634
Kurz WGW, La Rue TA (1975) Nitrogenase activity inRhizobia in absence of plant host Nature 256:407–408
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Meijer EM, Verseveld HW van, Beek EG van der, Stouthamer AH (1977) Energy conservation during aerobic growth inParacoccus denitrificans. Arch Microbiol 112:25–34
Nelson LM, Salminen SO (1982) Uptake hydrogenase activity and ATP formation inRhizobium leguminosarum bacteroids. J Bacteriol 151:989–995
Papa S (1967) Proton translocation reactions in the respiratory chain. Biochim Biophys Acta 185:316–331
Peck HD, Jr, Gest H (1956) A new procedure for assay of bacterial hydrogenases. J Bacteriol 73:70–80
Poole RK, Waring AI, Chance B (1979) The reaction of cytochromeo inEscherichia coli with oxygen. Biochem J 184:379–389
Poole RK, Sivaram A, Salmon I, Chance B (1982) Photolysis at very low temperature of CO liganded cytochrome oxidase (cytochromed) in oxygen-limitedEscherichia coli. FEBS Lett 141:237–241
Robson RL, Postgate JR (1980) Oxygen and hydrogen in biological nitrogen fixation. Ann Rev Microbiol 34:183–207
Schubert KR, Jennings NT, Evans HJ (1978) Hydrogen reactions of nodulated plants. II. Effects on dry matter accumulation and nitrogen fixation. Plant Physiol 61:398–401
Stam H, Verseveld HW van, Stouthamer AH (1983) Derepression of nitrogenase in chemostat cultures of the fast growingRhizobium leguminosarum. Arch Microbiol 135:199–204
Stouthamer AH (1984) Energy generation and hydrogen metabolism inRhizobium. In: Veeger C, Newton WE (eds) Advances in nitrogen fixation research. Proceedings of the 5th international symposium on nitrogen fixation. Noordwijkerhout. Nijhoff/Junk. The Hague, Pudoc, Wageningen, pp 189–199
Verseveld HW van, Stouthamer AH (1976) Oxidative phosphorylation inMicrococcus denistrificans. Calculation of the P/O ratio in growing cells. Arch Microbiol 107:241–247
Verseveld HW van, Boon JP, Stouthamer AH (1979) Growth yields and the efficiency of oxidative phosphorylation ofParacoccus denitrificans during two (carbon) substrate-limited growth. Arch Microbiol 121:213–223
Verseveld HW van, Krab K, Stouthamer AH (1981) Proton pump coupled to cytochromec inParacoccus denitrificans. Biochim Biophys Acta 635:525–534
Verseveld HW, van, Braster M, Boogerd FC, Chance B, Stouthamer AH (1983) Energetic aspects of growth ofParacoccus denitrificans: Oxygen-limitation and shift from anaerobic nitrate-limitation to aerobic succinate limitation. Arch Microbiol 135:229–236
Vries W de, Stouthamer AH (1968) Fermentation of glucose, lactose, mannitol and xylose byBifidobacteria. J Bacteriol 96:472–478
Zumft WG, Mortensen LE (1975) The nitrogen fixing complex of bacteria. Biochim Biophys Acta 416:1–52
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stam, H., van Verseveld, H.W., de Vries, W. et al. Hydrogen oxidation and efficiency of nitrogen fixation in succinate-limited chemostat cultures ofRhizobium ORS 571. Arch. Microbiol. 139, 53–60 (1984). https://doi.org/10.1007/BF00692712
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00692712