Summary
The thermal characteristics of flight muscle and liver pyruvate kinase (PK) from the batMyotis lucifugus have been investigated in an attempt to understand the molecular strategies associated with hibernation. PKs of the hibernating and normothermic state differ in essentially five ways:
-
1.
Qualitative differences exist in the isozymic patterns (Fig. 1), with hibernator muscle PK having fewer types than normothermic muscle PK, and hibernator and normothermic liver PKs differing in the arrangement of bands, not total number.
-
2.
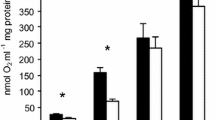
The specific activity of hibernator-PK is 1.5 to 2-fold higher than the normothermic enzyme (Figs. 2, 3, 5) which suggests the importance of this enzymic step at some point in the seasonal hibernating cycle of the bat.
-
3.
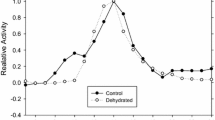
The PK found in a particular physiological state is that with the lowest thermal sensitivity (as indicated by reducedE a-values) over the temperature range characteristic of that state (Figs. 3, 5, 6, 7).
-
4.
Discontinuous Arrhenius plots are found for hibernator and normothermic tissue PKs, although the “critical temperature” (T c-value) is approximately 10°C lower for the hibernator enzyme (Figs. 3, 5).
-
5.
Temperature sensitivity is also noted in the binding of PEP and ADP to normothermic PK, although not in the hibernator enzyme (Figs. 4, 6, 7). The advantage of constant or fluctuating Q10-values is discussed in light of the biology of the bat.
Similar content being viewed by others
Abbreviations
- Ea :
-
activation energy
- FDP :
-
fructose-1,6-diphosphate
- PEP :
-
phosphoenol pyruvate
- PK :
-
pyruvate kinase
- Nl- andHL-:
-
normothermic and hibernator liver, respectively
- NM- andHM-:
-
normothermic and hibernator muscles, respectively
References
Behrisch, H.W.: Temperature and the regulation of enzyme activity in the hibernator. Isoenzymes of liver pyruvate kinase from the hibernating and non-hibernating Arctic ground squirrel. Canad. J. Biochem.52, 894–902 (1974)
Borgmann, U., Laidler, K.J., Moon, T.W.: Kinetics and thermodynamics of lactate dehydrogenases from beef heart, beef muscle and flounder muscle. Canad. J. Biochem.53, 1196–1206 (1975)
Borgmann, U., Moon, T.W.: A comparison of lactate dehydrogenases from an ectothermic and an endothermic animal. Canad. J. Biochem.53, 998–1004 (1975)
Brabec, M.J., McColloch, R.J.: Phosphofructokinase activity in the brown fat, liver, and heart of hibernating and homeothermic thirteen-lined ground squirrels (Citellus tridecemlineatus). Int. J. Biochem.1, 557–560 (1970)
Brush, A.H.: Response of isozymes to torpor in the bat,Eptesicus fuscus. Comp. Biochem. Physiol.27, 113–120 (1968)
Burlington, R.F.: Recent advances in intermediary metabolism of hibernating mammals. In: Hibernation hypothermia: Perspectives and challenges (eds. F.E. South, J.P. Hannon, J.R. Willis, E.T. Pengelley, N.R. Alpert), pp. 3–16. Amsterdam: Elsevier Publishing Co. 1972
Burlington, R.F., Sampson, J.H.: Distribution and activity of lactic dehydrogenase isozymes in tissues from a hibernator and a non-hibernator. Comp. Biochem. Physiol.25, 185–192 (1968)
Carmody, G.R., Fenton, M.B., Lee, D.S.: Variation of body weight and proteins in three Ontario populations of hibernatingMyotis lucifugus lucifugus (Le Conte) (Chiroptera: Vespertilionidae). Canad. J. Zool.49, 1535–1540 (1971)
Davis, D.J.: Disc electrophoresis. II. Method and application to human serum proteins. Ann. N.Y. Acad. Sci.121, 404–427 (1964)
Davis, W.H., Reite, O.B.: Responses of bats from temperate regions to changes in ambient temperature. Biol. Bull. Mar. Biol. Lab. (Woods Hole)132, 320–328 (1967)
Fenton, M.B.: Population studies ofMyotis lucifugus (Chiroptera: Vespertilionidae) in Ontario. Life Sci. Contr., R. Ont. Mus.77, 1–34 (1970)
Fenton, M.B.: Distribution and overwintering ofMyotis leibii andEptesicus fuscus (Chiroptera: Vespertilionidae) in Ontario. Life Sci. Occ. Pap., R. Ont. Mus.21, 1–8 (1972)
Groves, W. E., Davis, F.C., Sells, B.H.: Spectrophotometric determination of microgram quantities of protein without nucleic acid interference. Analyt. Biochem.22, 195–210 (1968)
Hayward, J.S., Lyman, C.P.: Nonshivering heat production during arousal from hibernation and evidence for the contribution of brown fat. In: Mammalian hibernation, III (eds. K.C. Fisher, A.R. Dawe, C.P. Lyman, E. Schönbaum, R.E. South, Jr.), pp. 346–355. London: Oliver and Boyd 1967
Henshaw, R.E.: Thermoregulation in bats. In: About bats. A chiropteran symposium (eds. B. H. Slaughter, D.W. Walton), pp. 188–232. Dallas: Southern Methodist University Press 1970
Hochachka, P.W., Somero, G.N.: Strategies of biochemical adaptation. Toronto: W.B. Saunders Co. 1973
Hock, R.J.: The metabolic rates and body temperatures of bats. Biol. Bull. Mar. Biol. Lab. (Woods Hole)101, 289–299 (1951)
Kayne, F.J.: Pyruvate kinase. In: The enzymes, 3rd ed., Vol. VIII (ed. P.D. Boyer), pp. 353–382. New York: Academic Press 1973
Kayne, F.J., Suelter, C.H.: The temperature-dependent conformational transitions of pyruvate kinase. Biochem.7, 1678–1684 (1968)
Low, P.S., Somero, G.N.: Temperature adaptation of enzymes: A proposed molecular basis for the different catalytic efficiencies of enzymes from ectotherms and endotherms. Comp. Biochem. Physiol.49B, 307–312 (1974)
Lyman, C.P.: Thermoregulation and metabolism in bats. In: Biology of bats, Vol. 1 (W.A. Wimsatt, ed.), pp. 301–330. New York: Academic Press 1970
Manwell, C., Kerst, K.V.: Possibilities of biochemical taxonomy of bats using hemoglobins, lactate dehydrogenases, esterases and other proteins. Comp. Biochem. Physiol.17, 741–754 (1966)
Moon, T.W., Borgmann, A.I.: Enzymes of the normothermic and hibernating bat,Myotis lucifugus: Metabolites as modulators of pyruvate kinase. J. comp. Physiol.107, 201–210 (1976)
Moon, T.W., Hochachka, P.W.: Temperature and enzyme activity in poikilotherms. Isocitrate dehydrogenases in rainbow trout liver. Biochem. J.123, 695–705 (1971)
Olsson, S.-O.R.: Comparative studies on the temperature dependence of lactate and malate dehydrogenases from a homeotherm, guinea pig (Cavia porcellus); two hibernators, hedgehog (Erinaceus europaeus) and bat (Nyctalus noctula); and two poikilotherms, frog (Rana temporaria) and cod (Gadus callarias). Comp. Biochem. Physiol.51B, 5–18 (1975)
Raison, J.K.: The influence of temperature-induced phase changes on the kinetics of respiratory and other membrane-associated enzyme systems. Bioenergetics4, 285–309 (1973)
Raison, J.K., Lyons, J.M.: Hibernation: Alteration of mitochondrial membranes as a requisite for metabolism at low temperatures. Proc. nat. Acad. Sci. (Wash.)68, 2092–2094 (1971)
Reite, O.B., Davis, W.H.: Thermoregulation in bats exposed to low ambient temperatures. Proc. Soc. expt. Biol. (N.Y.)121, 1212–1215 (1966)
Schloen, L.H., Kmiotek, E.H., Sallach, H.J.: Pyruvate kinase isozymes in adult tissues and eggs ofRana pipiens. Comparative studies on the properties of the different isozymes. Arch. Biochem. Biophys.164, 254–265 (1974)
Scrutton, M.C., Utter, M.F.: The regulation of glycolysis and gluconeogenesis in animal tissues. Ann. Rev. Biochem.37, 249–302 (1968)
Seubert, W., Schoner, W.: The regulation of pyruvate kinase. Curr. Top. Cell. Reg.3, 237–267 (1971)
Somero, G.N.: Pyruvate kinase variants of the Alaskan king-crab. Evidence for a temperature-dependent interconversion between two forms having distinct and adaptive kinetic properties. Biochem. J.114, 237–241 (1969)
Somero, G.N., Hochachka, P.W.: The effect of temperature on catalytic and regulatory functions of pyruvate kinases of the rainbow trout and the Antarctic fishTrematomus bernacchii. Biochem. J.110, 395–400 (1968)
Susor, W.A., Rutter, W.J.: Some distinctive properties of pyruvate kinase purified from rat liver. Biochem. biophys. Res. Commun.30, 14–20 (1968)
Tashima, L.S., Adelstein, S.J., Lyman, C.P.: Radioglucose utilization by active, hibernating and arousing ground squirrels. Amer. J. Physiol.218, 303–309 (1970)
Weber, G.: Regulation of pyruvate kinase. Adv. Enz. Reg.7, 15–40 (1969)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Borgmann, A.I., Moon, T.W. Enzymes of the normothermic and hibernating bat,Myotis lucifugus: Temperature as a modulator of pyruvate kinase. J Comp Physiol B 107, 185–199 (1976). https://doi.org/10.1007/BF00691225
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00691225