Abstract
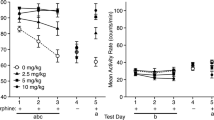
The role of β-endorphin as a possible mediator in the reinforcing properties of opiates was investigated using a conditioned place preference paradigm. Heroin, a synthetic opiate known to have reinforcing properties, produced a strong preference for an environment previously paired with heroin injection at all doses tested (0.25, 0.5, 1.0, 2.0 mg/kg SC). No such place preference was observed following saline injections. Rats also showed dose-dependent place preference for the environment paired with β-endorphin when injected intracerebroventricularly (significant dose was 2.5 μg). At higher doses (5.0 and 10.0 μg) rats showed no preference for the paired environment, but were catatonic. Pretreatment with naloxone (0.04, 0.2, 1.0 mg/kg SC) attenuated the rewarding effect of β-endorphin (2.5 μg) at all doses tested. The lowest dose of naloxone which had no aversive effect when tested alone could also significantly block the positive effect of β-endorphin. The reinforcing dose of β-endorphin (2.5 μg) also produced an increase in locomotor activity, when tested in photocell cages. This suggests that the hyperactivity induced by β-endorphin may contribute to the preference for an environment previously paired with the same drug. The reinforcing effect of β-endorphin is most probably mediated by the mu and/or delta opioid subtype receptor, since β-endorphin has a high affinity for these receptors. These results demonstrate positive reinforcing properties of β-endorphin in the central nervous system.
Similar content being viewed by others
References
Amalric M, Koob GF (1985) Low doses of methylnaloxonium in the nucleus accumbens antagonize hyperactivity induced by heroin in the rat. Pharmacol Biochem Behav 23:411–415
Bardo MT, Miller JS, Neisewander JL (1984) Conditioned place preference with morphine: The effects of extinction training on the reinforcing CR. Pharmacol Biochem Behav 21:545–543
Belluzzi JD, Stein L (1977) Enkephalin may mediate euphoria and drive-reduction reward. Nature 266:556–558
Blander A, Hunt T, Blair R, Amit Z (1984) Conditioned place preference: An evaluation of morphine positive reinforcing properties. Psychopharmacology 84:124–127
Bloom FE, Segal D, Ling N, Guillemin R (1976) Endorphins: profound behavioral effects in rats suggest new etiological factors in mental illness. Science 194:630–632
Bozarth MA, Wise RA (1981a) Heroin reward is dependent on a dopaminergic substrate. Life Sci 29:1881–1886
Bozarth MA, Wise RA (1981b) Intracranial self-administration of morphine into the ventral tegmental area in rats. Life Sci 28:551–555
Broekkamp CL, Phillips AG, Cools AR (1979) Facilitation of self-stimulation behavior following intracerebral microinjections of opioids into the ventral tegmental area. Pharmacol Biochem Behav 11:289–295
Browne RG, Derrington DC, Segal DS (1979) Comparison of opiate — and opioid — peptide induced immobility. Life Sci 24:933–942
Cappell H, LeBlanc AE, Endrenyi L (1973) Aversive conditioning by psychoactive drugs: effects of morphine, alcohol and chlordiazepoxide. Psychopharmacology 29:239–246
Elde R, Hokfelt T, Johansson O, Terenius L (1976) Immunohistochemical studies using antibodies to leucine-enkephalin: initial observations on the nervous system of the rat. Neuroscience 1:349–351
Goeders NE, Lane JD, Smith JE (1984) Self-administration of methionine enkephalin into the nucleus accumbens. Pharmacol Biochem Behav 20:451–455
Inturrisi CE, Schultz M, Shin S, Umans JG, Angel L, Simon EJ (1983) Evidence from opiate binding studies that heroin acts through its metabolites. Life Sci 33:773–776
Jacquet YF, Marks N (1976) The c-fragment of β-lipotropin: An endogenous neuroleptic or antipsychogen? Science 194:632–635
Katz RJ, Gormezano G (1979) A rapid inexpensive technique for assessing the reinforcing effects of opiate drugs. Pharmacol Biochem Behav 11:231–233
Kelley AE, Stinus L, Iversen SD (1980) Interaction betweend-alamet-enkephalin, A 10 dopaminergic neurones and spontaneous behavior in the rat. Behav Brain Res 1:3–24
Kelsey JE, Belluzzi JD, Stein L (1984) Does naloxone suppress self-stimulation by decreasing reward or by increasing aversion? Brain Res 307:55–59
Kumar R (1972) Morphine dependence in rats: Secondary reinforcement from environmental stimuli. Psychopharmacologia 25:332–338
Loh HH, Tseng LF, Wei ET, Li CH (1976) β-endorphin is a potent analgesic agent. Proc Natl Acad Sci USA 73:2835–2898
Mucha FR, Iversen SD (1984) Reinforcing properties of morphine and naloxone revealed by conditioned place preferences: A procedural examination. Psychopharmacology 82:241–247
Mucha RF, Herz A (1985) Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology 86:274–280
Mucha RF, Van der Kooy D, O'Shaughressy M, Bucenieks P (1982) Drug reinforcement studied by use of place conditioning in rat. Brain Res 243:91–105
Mucha RF, Millan MJ, Herz A (1985) Aversive properties of naloxone in non-dependent (naive) rats may involve blockade of central β-endorphin. Psychopharmacology 86:281–285
Olds ME (1982) Reinforcing effects of morphine in the nucleus accumbens. Brain Res 237:429–440
Olds ME, Williams KN (1980) Self-administration ofd-ala 2-metenkephalinamide at hypothalamic self-stimulation sites. Brain Res 194:155–170
Phillips AG, LePiane FG (1980) Reinforcing effects of morphine microinjection into the ventral tegmental area. Pharmacol Biochem Behav 12:965–968
Phillips AG, LePiane FG (1982) Reward produced by microinjection ofd-ala-met-enkephalinamide into ventral tegmental area. Behav Brain Res 5:225–229
Phillips AG, LePiane FG, Fibiger HC (1983) Dopaminergic mediation of reward produced by direct injection of enkephalin into the vental tegmental area of the rat. Life Sci 33:2505–2511
Rossi NA, Reid LD (1976) Affective states associated with morphine injection. Physiol Psychol 4:269–274
Schenk S, Ellison F, Hunt T, Amit Z (1985) An examination of heroin conditioning in preferred and non preferred environments and in differentially housed mature and immature rats. Pharmacol Biochem Behav 22:215–220
Segal DS, Browne RG, Arnsten A, Derrington DC, Bloom FE, Guillemin R, Ling N (1979) Characteristics of β-endorphin induced behavioral activation and immobilization. In: Usdin E, Bunney WE, Kline NS (eds) Endorphins in mental health research. MacMillan, London, pp 307–324
Sherman JE, Pickman C, Rice A, Liebeskind JC, Homan EW (1980) Rewarding and aversive effects of morphine: Temporal and pharmacological properties. Pharmacol Biochem Behav 13:501–505
Spyraki C, Fibiger HC, Phillips AG (1983) Attenuation of heroin reward in rats by disruption of the mesolimbic dopamine system. Psychopharmacology 79:278–283
Stapleton JM, Lind MD, Merriman VJ, Bozarth MA, Reid LD (1979) Affective consequences and subsequent effects on morphine self-administration of d-ala2-methionine enkephalin. Physiol Psychol 7:146–152
Stein L, Belluzzi JD (1978) Brain endorphins and the sense of well-being: A psychobiological hypothesis. Adv Biochem Psychopharm 18:299–311
Stinus L, Koob GF, Ling N, Bloom FE, LeMoal M (1980) Locomotor activation induced by infusion of endorphins into the ventral tegmental area: Evidence for opiate-dopamine interactions. Proc Natl Acad Sci USA 77:2323–2327
Swerdlow NR, Koob GF (1984) Restrained rats learn amphetamine conditioned locomotion, but not place preference. Psychopharmacology 84:163–166
Tseng LF, Nei ET, Loh HH, Choh HL (1980) β-endorphin: central sites of analgesia, catalepsy and body temperature changes in rats. J Pharmacol Exp Ther 214:328–332
Vaccarino FJ, Bloom FE, Koob GF (1985) Blockade of nucleus accumbens opiate receptors attenuates intravenous heroin reward in the rat. Psychopharmacology 86:37–42
Van der Kooy D, Mucha RF, O'Shaughnessey M, Bucenieks P (1982) Reinforcing effects of brain microinjections of morphine revealed by conditioned place preference. Brain Res 243:107–117
Way EL, Adler TK (1960) The pharmacologic implications of the fate or morphine and its surrogate. Pharmacol Rev 12:383–446
Wolfswinkel L, Van Ree JM (1985) Differential effect of naloxone on food and self-stimulation rewarded acquisition of a behavioral response pattern. Pharmacol Biochem Behav 23:199–202
Zukin RS, Zukin SR (1984) The case for multiple opiate receptors. TINS 7:160–164
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Amalric, M., Cline, E.J., Martinez, J.L. et al. Rewarding properties of β-endorphin as measured by conditioned place preference. Psychopharmacologia 91, 14–19 (1987). https://doi.org/10.1007/BF00690919
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00690919