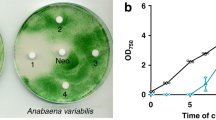
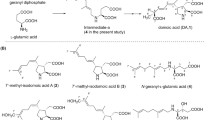
Abstract
Gabaculin (3-amino 2,3-dihydrobenzoic acid) inhibited the growth of cyanobacteria but not of other prokaryotes. Exposure of growing cultures ofSynechococcus 6301 to 50 μM gabaculin resulted in an immediate and complete inhibition of the synthesis of chlorophylla and phycocyanin. With 8 μM gabaculin, tetrapyrrole synthesis was suppressed for approximately 10 h and then resumed at a lower rate than in untreated organisms. The effect of 50 μM gabaculin was reversed by transferring organisms to inhibitor-free medium; chlorophylla synthesis began within 5 h and exponential growth was re-established after about 25 h. Compared with 4,6-dioxoheptanoic acid (DA) and laevulinic acid (LA), gabaculin was a much more potent inhibitor of tetrapyrrole synthesis inSynechococcus 6301. The catalytic activity of δ-aminolaevulinic acid (ALA) dehydratase in vitro was inhibited by DA and LA but not by 1 mM gabaculin. However, the specific activity of the dehydratase was much lower in organisms exposed to the inhibitor for 36 h. Growing cultures and cell suspensions ofSynechococcus 6301 exposed to DA excreted appreciable quantities of ALA. In contrast, relatively small amounts of ALA accumulated in the presence of gabaculin alone and this inhibitor blocked the excretion of ALA caused by DA. This suggests that the primary effect of gabaculin is the specific inhibition of the C5 pathway for the biosynthesis of ALA.
Similar content being viewed by others
Abbreviations
- ALA:
-
δ-aminolaevulinic acid
- DA:
-
4,6-dioxoheptanoic acid
- LA:
-
laevulinic acid
- GABA:
-
γ-aminobutyric acid
References
Aaronson S, Baker H (1959) A comparative biochemical study of two species ofOchromonas. J Protozool 6:282–284
Allen MB (1959) Studies withCyanidium caldarium an anomalously pigmented chlorophyte. Arch Mikrobiol 32:270–277
Allen MM, Smith AJ (1969) Nitrogen chlorosis in blue-green algae. Arch Mikrobiol 69:114–120
Andersen T, Briseid T, Nesbakken T, Ormerod J, Sirevag R, Thorud M (1983) Mechanisms of synthesis of 5-aminolevulinate in purple, green and blue-green bacteria. FEMS Microbiol Lett 19:303–306
Beale SI (1970) The biosynthesis of δ-aminolevulinic acid inChlorella. Plant Physiol 45:504–506
Beale SI (1978) δ-Aminolevulinic acid in plants: Its biosynthesis, regulation and role in plastid development. Ann Rev Plant Physiol 29:95–120
Beauclerk AAD, Smith AJ (1978) Transport ofd-glucose and 3-O-methyld-glucose in the cyanobacteriaAphanocapsa 6714 andNostoc strain MAC. Eur J Biochem 82:187–197
Castelfranco PA, Beale SI (1983) Chlorophyll biosynthesis: Recent advances and areas of current interest. Ann Rev Plant Physiol 34:241–278
Granick S, Beale SI (1978) Hemes, chlorophylls and related compounds: Biosynthesis and metabolic regulation. In: Meister A (ed) Advances in enzymology, vol 46. Wiley, New York, pp 33–203
Hill CM, Pearson SH, Smith AJ, Rogers LJ (1985) Inhibition of chlorophyll synthesis inHordeum vulgare by 3-amino 2,3-dihydrobenzoic acid (gabaculin). Bioscience Reports 5:775–781
Hodge JE, Hofreiter BT (1962) Determination of reducing sugars and carbohydrates. In: Whistler RL, Wolform ML (eds) Methods in carbohydrate biochemistry, vol 1. Academic Press, London, pp 380–394
Kannangara CG, Gough SP (1978) Biosynthesis of δ-aminolevulinate in greening barley leaves: Glutamate 1-semialdehyde aminotransferase. Carlsberg Res Commun 43:185–194
Kannangara CG, Schouboe A (1985) Biosynthesis of δ-aminolevulinate in greening barley leaves. VII. Glutamate 1-semialdehyde accumulation in gabaculine treated leaves. Carlsberg Research Commun 50:179–191
Kipe-Nolt JA, Stevens SE, Stevens CLR (1978) Biosynthesis of δ-aminolevulinic acid by blue-green algae (cyanobacteria). J Bacteriol 135:286–288
Kobayashi K, Miyazawa S, Terahara A, Mishima H, Kurihara H (1976) Gabaculine: δ-Aminobutyrate aminotransferase inhibitor of microbial origin. Tetrahedron Lett 7:537–540
Lascelles J (1956) The synthesis of porphyrins and bacteriochlorophyll by cell suspensions ofRhodopseudomonas spheroides. Biochem J 62:78–93
Laycock MV, Wright JLC (1981) The biosynthesis of phycocyanobilin inAnacystis nidulans. Phytochemistry 20:1265–1268
Lewis NG, Walter JA, Wright JLC (1984) Tetrapyrrole biosynthesis inAnacystis nidulans; incorporation of [1-13C]-, [2-13C]-, [1,2-13C]-and [2-13C, 2-2H3]-acetate. Phytochemistry 23:1611–1616
Lowry DH, Rosebrough NJ, Farr AC, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
McKie J, Lucas C, Smith AJ (1981) δ-Aminolaevulinate biosynthesis in the cyanobacteriumSynechococcus 6301. Phytochemistry 20:1547–1549
Mauzerall D, Granick S (1956) The occurrence and determination of δ-aminolevulinic acid and porphobilinogen in urine. J Biol Chem 219:435–446
Meisch H-U, Maus R (1983) Untersuchungen zur synthesen und biologischen Bedeutung von glutaminsaure-1-semialdehyd als Vorstufe der Chlorophylle. Z Naturforsch 38c:563–570
Meisch H-U, Reinle W, Wolf U (1985) The problem of 4,5-dioxovaleric acid as a precursor of 5-aminolevulinic acid and chlorophyll in green algae. Biochim Biophys Acta 841:319–322
Meller E, Gassman ML (1981) The effects of levulinic acid and 4,6-dioxoheptanoic acid on the metabolism of etiolated and greening barley leaves. Plant Physiol 67:728–732
Ormerod JG, Ormerod KS, Gest H (1961) Light dependent utilisation of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochim Biophys 94:449–463
Ougham JH, Taylor DG, Trudgill PW (1983) Camphor revisited: Involvement of a unique monooxygenase in metabolism of 62-1 acid byPseudomonas putida. J Bacteriol 153:140–152
Rando RR (1977) Mechanism of the irreversible inhibition of γ-aminobutyric acid-α-ketoglutaric acid transaminase by the neuro toxin gabaculine. Biochemistry 16:4604–4610
Shemin D (1970) δ-Aminolevulinic acid dehydratase (Rhodopseudomonas spheroides). In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol XVIIA. academic Press, New York, pp 205–211
Shibata K (1959) Spectrophotometry of translucent biological materials — opal glass transmission method. In: Glick D (ed) Methods of biochemical analysis, vol 7. Interscience Publishing Co. Inc., New York, pp 77–109
Smith AJ, London J, Stanier RY (1967) Biochemical basis of obligate autotrophy in blue-green algae and thiobacilli. J Bacteriol 94:972–983
Sorokin C, Krauss RW (1958) The effects of light intensity on the growth rates of green algae. Plant Physiol 33:109–113
Weed LL, Longfellow D (1954) Morphological and biochemical changes induced by copper in a population ofEscherichia coli. J Bacteriol 67:27–33
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hoult, R.C., Rees, D., Rogers, L.J. et al. Specific inhibition of tetrapyrrole biosynthesis by 3-amino 2,3-dihydrobenzoic acid (gabaculin) in the cyanobacteriumSynechococcus 6301. Arch. Microbiol. 146, 57–62 (1986). https://doi.org/10.1007/BF00690159
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00690159