Abstract
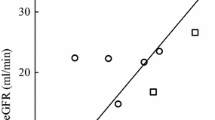
The present study was undertaken to evaluate in children the plasma pharmacokinetics of free carboplatin given at different doses and schedules and to evaluate the inter- and intrapatient variability and the possible influence of schedule on drug exposure. A total of 35 children (age range, 1–17 years) with malignant tumors were studied. All patients had normal renal function (creatinine clearance corrected for surface body area, above 70 ml min−1 m−2; range, 71–151 ml min−1 m−2) and none had renal involvement by malignancy. Carboplatin was given at the following doses and schedules: 175, 400, 500, and 600 mg/m2 given as a 1-h infusion; 1,200 mg/m2 divided into equal doses and infused over 1 h on 2 consecutive days; and 875 and 1,200 mg/m2 given as a 5-day continuous infusion. A total of 57 courses were studied. Carboplatin levels in plasma ultrafiltrate (UF) samples were measured both by high-performance liquid chromatography and by atomic absorption spectrophotometry. Following a 1-h infusion, carboplatin free plasma levels decayed biphasically; the disappearance half-lives, total body clearance, and apparent volume of distribution were similar for different doses. In children with normal renal function as defined by creatinemia and blood urea nitrogen (BUN) and creatinine clearance, we found at each dose studied a limited interpatient variability of the peak plasma concentration (Cmax) and the area under the concentration-time curve (AUC) and a linear correlation between the dose and both Cmax (r=0.95) and AUC (r=0.97). The mean value ± SD for the dose-normalized AUC was 13±2 min m2 l−1 (n=57). The administration schedule does not seem to influence drug exposure, since prolonged i.v. infusion or bolus administration of 1,200 mg/m2 achieved a similar AUC (13.78±2.90 and 15.05±1.44 mg ml−1 min, respectively). In the nine children studied during subsequent courses a limited interpatient variability was observed and no correlation (r=0.035) was found between AUC and subsequent courses by a multivariate analysis of dose, AUC, and course number. The pharmacokinetic parameters were similar to those previously reported in adults; however, a weak correlation (r=0.52,P=0.03) between carboplatin total body clearance and creatinine clearance varying within the normal range was observed. A dosing formula appears unnecessary in children with normal renal function since a generally well-predictable free carboplatin AUC is achieved following a given dose.
Similar content being viewed by others
References
Bacha DM, Caparros-Sison B, Allen JA, Walker R, Tan CT (1986) Phase I study of carboplatin (CBDCA) in children with cancer. Cancer Treat Rep 70: 865
Calvert AH, Harland SJ, Newell DR, Siddik ZH, Jones AC, McElwain TJ, Rajus S, Wiltshaw E, Smith IE, Baker JM, Peckham MJ, Harrap KR (1982) Early clinical studies withcis-diamminel,1-cyclobutane platium (II). Cancer Chemother Pharmacol 9:140
Calvert AH, Newell DR, Gumbrell LA, O'Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME, Wiltshaw E (1989) Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 7: 1748
Canetta R, Bragman K, Smaldone L, Rozencweig M (1988) Carboplatin: current status and future prospects. Cancer Treat Rev 15 [Suppl B]: 17
Curt GA, Grygiel JJ, Corden BJ, Ozols RF, Weiss RB, Tell DT, Myers CE, Collins JM (1983) Phase I and pharmacokinetic study of diamminecyclobutane-dicarboxylatoplatinum (NSC241240). Cancer Res 43: 4470
Daugaard G, Rossing N, Rorth M (1988) Effects of cisplatin on different measures of glomerular function in the human kidney with special emphasis on high-dose. Cancer Chemother Pharmacol 21: 163
Doz F, Brughieres L, Bastian G, Quintana E, Lemerle J, Zucker JM (1990) Clinical trial and pharmacokinetics of carboplatin 560 mg/m2 in children. Med Pediatr Oncol 18: 459
Egorin MJ, Van Echo DA, Tipping SJ, Olman EA, Witacre MY, Thompson BW, Aisner J (1984) Pharmacokinetics and dosage reduction ofcis-diammine-(1.1-cyclobutanedicarboxylato) platinum in patients with impaired renal function. Cancer Res 44: 5432
El-Yazigi A, Al-Saleh I (1986) Rapid determination of platinum by flameless atomic absorption spectrophotometry following the administration of cisplatin to cancer patients. Ther Drug Monit 8: 318
Elferik F, Van der Vijgh WJF, Klein I, Vermorken JB, Gall HE, Pinedo HM (1987) Pharmacokinetics of carboplatin after iv administration. Cancer Treat Rep 71: 1231
Gaver RC, Colombo N, Green MD, George AM, Deeb G, Morris AD, Canetta RM, Speyer JL, Farmen RH, Muggia FM (1988) The disposition of carboplatin in ovarian cancer patients. Cancer Chemother Pharmacol 22: 263
Gaver RC, Deeb G (1986) High-performance liquid chromatographic procedures for the analysis of carboplatin in human plasma and urine. Cancer Chemother Pharmacol 16:201
Gaynon PS, Ettinger LJ, Moel D, Siegel SE, Baum ES, Krivit W, Hammond GD (1987) Pediatric phase I trial of carboplatin: a Children Cancer Study Group report. Cancer Treat Rep 71: 1039
Gore ME, Calvert AH, Smith IE (1987) High dose of carboplatin in the treatment of lung cancer and mesothelioma: a phase I dose escalation study. Eur J Cancer Clin Oncol 23: 1391
Gormley PE, Bull JM, LeRoy AF, Cysyr R (1979) Kinetics ofcis-dichlorodiammineplatinum. Clin Pharmacol Ther 25: 351
Harland SJ, Newell DR, Siddick ZH, Chadwich R, Calvert AH, Harrap KR (1984) Pharmacokinetics ofcis-diammine-1,1-cyclobutane dicarboxylato platinum(II) in patients with normal and impaired renal function. Cancer Res 44: 1693
Knox RJ, Fridslos F, Lydall DA, Roberts JJ (1986) Mechanism of cytotoxicity of anticancer platinum drugs: evidence thatcis-diamminedichloroplatinum(II) andcis-diammine-(1,1-cyclobutanedicarboxylato) platinum(II) differ only in the kinetics of their interaction with DNA. Cancer Res 46: 1972
Koeller JM, Trump DL, Tutsch KD, Earhart RH, Davis TE, Tormey DC (1986) Phase I clinical trial and pharmacokinetics of carboplatin (NSC 241240) by single monthly 30-minute infusion. Cancer 57: 222
Lee EJ, Egorin MJ, Van Echo DA, Cohen AE, Tait N, Schiffer CA (1988) Phase I and pharmacokinetic trial of carboplatin in refractory adult leukemia. J Natl Cancer Inst 80: 131
Leyvraz S, Ohnuma T, Lassus M, Holland FJ (1985) Phase I study of carboplatin in patients with advanced cancer, intermittent intravenous bolus, and 24-hour infusion. J Clin Oncol 3: 1385
Loehrer PJ, Einhorn LH (1984) Cisplatin. Ann Intern Med 100: 704
Madden T, Sunderland M, Santana VM, Rodman JH (1992) The pharmacokinetics of high-dose carboplatin in pediatric patients with cancer. Clin Pharmacol Ther 51: 701
Muggia FM (1989) Overview of carboplatin: replacing complementing and extending the therapeutic horizons of cisplatin. Semin Oncol 16: 7
Mulder POM, De Vries EGE, Uges DRA, Scaf AHJ, Sleiifer DTH, Mulder NH (1990) Pharmacokinetics of carboplatin at a dose of 750 mg/m2 divided over three consecutive days. Br J Cancer 61: 460
Newell DR, Eeles RA, Gumbrell LA, Boxall FE, Horwinch A, Calvert AH (1989) Carboplatin and etoposide pharmacokinetics in patients with testicular teratoma. Cancer Chemother Pharmacol 23: 367
Newell DR, Siddik ZH, Gumbrell LA, Boxall FE, Gore ME, Smith IE, Calvert AH (1987) Plasma free platinum pharmacokinetics in patients treated with high dose carboplatin. Eur J Cancer Clin Oncol 23: 1399
Oguri S, Sakakibara T, Mase H, Shimizu T, Ishikawa K (1988) Clinical pharmacokinetics of carboplatin. J Clin Pharmacol 28: 208
Reece PA, Stafford I, Russell J, Grantley G (1986) Reduced ability to clear ultrafilterable platinum with repeated courses of cisplatin. J Clin Oncol 4: 1392
Reed E, Kohn KW (1990) Platinum analogues. In: Chabner BA (ed) Cancer chemotherapy. J. B. Lippincott, Philadelphia, pp 465–490
Riccardi R, Riccardi A, Di Rocco C, Carelli G, Tartaglia RL, Lasorella A, Servidei T, Mastrangelo R (1992) Cerebrospinal fluid pharmacokinetics of carboplatin in children with brain tumors. Cancer Chemother Pharmacol 30: 21
Sasaki Y, Tamura T, Enguchi K, Shinkai T, Fujiwara Y, Fukuda M, Ohe Y, Bungo M, Horichi N, Niimi S, Minato K, Nakagawa K, Sajo N (1989) Pharmacokinetics of (glycolato-0,0′)-diammine platinum(II), a new platinum derivative, in comparison with cisplatin and carboplatin. Cancer Chemother Pharmacol 23: 243
Shea TC, Flaherty M, Elias A, Eder JP, Antman K, Begg C, Schnipper L, Frei E, Henner WD (1989) Phase I clinical and pharmacokinetic study of carboplatin and autologous bone marrow support. J Clin Oncol 7: 651
Takahashi K, Seki T, Nishikawa K, Minamide S, Twabuchi M, Ono M, Nagamine S, Horinishi H (1985) Antitumour activity and toxicity of serum protein-bound platinum formed from cisplatin. Jpn J Cancer Res 76: 68
Van der Vijgh WJF (1991) Clinical pharmacokinetics of carboplatin. Clin Pharmacokinet 21: 242
Van Echo DA, Egorin MJ, Aisner J (1989) The pharmacology of carboplatin. Semin Oncol 16 [Suppl 5]: 1
Author information
Authors and Affiliations
Additional information
Supported by the Associazione Italiana per la Ricerca sul Cancro (A.I.R.C.)
Rights and permissions
About this article
Cite this article
Riccardi, R., Riccardi, A., Lasorella, A. et al. Clinical pharmacokinetics of carboplatin in children. Cancer Chemother. Pharmacol. 33, 477–483 (1994). https://doi.org/10.1007/BF00686504
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00686504