Abstract
Purpose
The Calvert formula was derived from the study among patients with glomerular filtration rates (GFRs) of 33–135 ml/min, and it remains unclear whether the formula can be used to calculate optimal and safe dosages of carboplatin in patients with severe renal insufficiency. We evaluated the utility of this formula in patients with severe renal insufficiency.
Methods
For pharmacokinetic analysis, we studied nine adult Japanese patients with advanced cancer who had an estimated GFR of lower than 30 ml/min/1.73 m2, as calculated by the Japanese equation for estimating GFR, or who were receiving hemodialysis. The dose of carboplatin was calculated with the Calvert formula, in which GFR was measured by inulin clearance or was assumed to be 0 in patients requiring hemodialysis. Hemodialysis was started 23 h after the end of carboplatin infusion.
Results
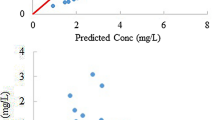
Although there was a significant correlation between the estimated and measured carboplatin clearance, the estimated clearance was consistently higher than the measured clearance [mean prediction error ± standard deviation = 41.0 ± 26.3 %] in all seven patients with renal insufficiency (GFR, median 21.4, range 7.8–31.4 ml/min) and in the two hemodialysis patients. Actual areas under the concentration–time curve (AUC) (mg/ml min) were 5.4, 5.7, 6.2, and 9.0 for the four patients with a target AUC (mg/ml min) of 5; 5.7, 6.2, and 7.1 for the three patients with a target AUC (mg/ml min) of 4; and 5.1 and 8.7 for the two hemodialysis patients with a target AUC (mg/ml min) of 5. The measured clearance of carboplatin ranged from 23.0 to 51.3 ml/min in the seven patients not receiving hemodialysis. The pre-hemodialysis carboplatin clearance in the hemodialysis patients was 20.5 and 11.1 ml/min, respectively.
Conclusion
For adult patients with severe renal insufficiency, the Calvert formula causes carboplatin overdosing by overestimating the carboplatin clearance.



Similar content being viewed by others
References
Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB et al (1999) Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet 354:93–99
Calvert AH, Newell DR, Gumbrell LA, O’Reilly S, Burnell M, Boxall FE et al (1989) Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 7:1748–1756
Ando Y, Shimokata T, Yasuda Y, Hasegawa Y (2014) Carboplatin dosing for adult Japanese patients. Nagoya J Med Sci 76:1–9
Oguri T, Shimokata T, Inada M, Ito I, Ando Y, Sasaki Y et al (2010) Pharmacokinetic analysis of carboplatin in patients with cancer who are undergoing hemodialysis. Cancer Chemother Pharmacol 66:813–817
Chatelut E, Rostaing L, Gualano V, Vissac T, De Forni M, Ton-That H et al (1994) Pharmacokinetics of carboplatin in a patient suffering from advanced ovarian carcinoma with hemodialysis-dependent renal insufficiency. Nephron 66:157–161
Motzer RJ, Niedzwiecki D, Isaacs M, Menendez-Botet C, Tong WP, Flombaum C et al (1990) Carboplatin-based chemotherapy with pharmacokinetic analysis for patients with hemodialysis-dependent renal insufficiency. Cancer Chemother Pharmacol 27:234–238
Watanabe M, Aoki Y, Tomita M, Sato T, Takaki Y, Kato N et al (2002) Paclitaxel and carboplatin combination chemotherapy in a hemodialysis patient with advanced ovarian cancer. Gynecol Oncol 84:335–338
Jeyabalan N, Hirte HW, Moens F (2000) Treatment of advanced ovarian carcinoma with carboplatin and paclitaxel in a patient with renal failure. Int J Gynecol Cancer 10:463–468
Egorin MJ, Van Echo DA, Tipping SJ, Olman EA, Whitacre MY, Thompson BW et al (1984) Pharmacokinetics and dosage reduction of cis-diammine (1,1-cyclobutanedicarboxylato) platinum in patients with impaired renal function. Cancer Res 44:5432–5438
English MW, Lowis SP, Peng B, Boddy A, Newell DR, Price L et al (1996) Pharmacokinetically guided dosing of carboplatin and etoposide during peritoneal dialysis and haemodialysis. Br J Cancer 73:776–780
Takezawa K, Okamoto I, Fukuoka M, Nakagawa K (2008) Pharmacokinetic analysis of carboplatin and etoposide in a small cell lung cancer patient undergoing hemodialysis. J Thorac Oncol 3:1073–1075
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K et al (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53:982–992
Horio M, Yasuda Y, Takahara S, Imai E, Watanabe T, Matsuo S (2010) Comparison of a simple and a standard method for inulin renal clearance. Clin Exp Nephrol 14:427–430
Screnci D, Er HM, Hambley TW, Galettis P, Brouwer W, McKeage MJ (1997) Stereoselective peripheral sensory neurotoxicity of diaminocyclohexane platinum enantiomers related to ormaplatin and oxaliplatin. Br J Cancer 76:502–510
Sheiner LB, Beal SL (1981) Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 9:503–512
Carmichael J, Allerheiligen S, Walling J (1996) A phase I study of gemcitabine and carboplatin in non-small cell lung cancer. Semin Oncol 23(Suppl 10):55–59
Leijen S, Veltkamp SA, Huitema AD, van Werkhoven E, Beijnen JH, Schellens JH (2013) Phase I dose-escalation study and population pharmacokinetic analysis of fixed dose rate gemcitabine plus carboplatin as second-line therapy in patients with ovarian cancer. Gynecol Oncol 130:511–517
Thomas HD, Porter DJ, Bartelink I, Nobbs JR, Cole M, Elliott S et al (2002) Randomized cross-over clinical trial to study potential pharmacokinetic interactions between cisplatin or carboplatin and etoposide. Br J Clin Pharmacol 53:83–91
Newell DR, Eeles RA, Gumbrell LA, Boxall FE, Horwich A, Calvert AH (1989) Carboplatin and etoposide pharmacokinetics in patients with testicular teratoma. Cancer Chemother Pharmacol 23:367–372
Obasaju CK, Johnson SW, Rogatko A, Kilpatrick D, Brennan JM, Hamilton TC et al (1996) Evaluation of carboplatin pharmacokinetics in the absence and presence of paclitaxel. Clin Cancer Res 2:549–552
Shimokata T, Ando Y, Yasuda Y, Hamada A, Kawada K, Saito H et al (2010) Prospective evaluation of pharmacokinetically guided dosing of carboplatin in Japanese patients with cancer. Cancer Sci 101:2601–2605
Tsuda A, Ishimura E, Ohno Y, Ichii M, Nakatani S, Machida Y et al (2014) Poor glycemic control is a major factor in the overestimation of glomerular filtration rate in diabetic patients. Diabetes Care 37:596–603
Acknowledgments
We are deeply indebted to Ms. Yuka Murasaki and Miki Sagou for their professional assistance in this study. This study was partly supported by the National Cancer Center Research and Development Fund [23-A-16]. Yoshinari Yasuda and Yoshinori Hasegawa have received research Grants from Pfizer Co. Ltd. and a speaker honorarium from Pfizer Co. Ltd. Yuichi Ando has received research Grants from Nippon Kayaku Co. Ltd. and a speaker honorarium from Pfizer Co. Ltd.
Conflict of interest
All other authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oguri, T., Shimokata, T., Ito, I. et al. Extension of the Calvert formula to patients with severe renal insufficiency. Cancer Chemother Pharmacol 76, 53–59 (2015). https://doi.org/10.1007/s00280-015-2769-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2769-9