Abstract
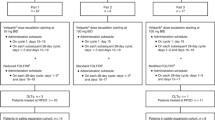
Biochemical modulation can increase the efficacy of 5-fluorouracil (5-FU). Pizzorno et al. have previously shown that brequinar, a de novo pyrimidine synthesis inhibitor, enhances the antitumor effect of 5-FU in vivo [Cancer Res 52: 1660–1665, 1992]. On the basis of their data, we conducted a phase I study of brequinar in combination with 5-FU in patients with refractory solid tumors. The initial dose (100 mg/m2) of brequinar was raised in 100-mg/m2 increments in cohorts of three assessable patients. The initial dose of 5-FU was 500 mg/m2, but escalation was allowed in patients who showed no significant toxic reaction. Brequinar was administered over 1 h and 5-FU over 2 h starting 18–20 h after the initiation of infusion of brequinar. Treatments were repeated weekly. Responses were evaluated after 4 weeks (one course) and then every 8 weeks thereafter. Pharmacokinetics of brequinar and determination of plasma uridine levels were performed in at least three patients at each dose level. Of the 25 patients registered in the study, 21 were assessable for toxicity studies. The dose of brequinar was escalated up to 600 mg/m2. In addition, the dose of 5-FU was increased to 600 mg/m2 as a result of a lack of a significant toxic reaction in the first nine patients. No objective responses were observed. One patient developed grade 3 stomatitis, and one developed grade 3 esophagitis at the 400 and 600 mg/m2 dose of brequinar, respectively. Brequinar produced a dosedependent decrease in plasma uridine levels at doses up to 500 mg/m2. No additional decrease in plasma uridine occurred with higher doses of brequinar, thus suggesting a plateau effect. This observation prompted us to terminate the study before reaching the maximum tolerated dose of brequinar. Our data indicate that brequinar in doses≥400 mg/m2 results in significant biochemical modulation. The lack of toxicity seen at these doses of brequinar suggests that the initial dose of the effector agent 5-FU should be increased in future studies.
Similar content being viewed by others
Abbreviations
- 5-FU :
-
5-Fluorouracil
- PALA :
-
N-(phosphonacetyl)-l-aspartic acid
- UXP :
-
total uridine nucleotide
- AGC :
-
absolute granulocyte count
- MTD :
-
maximum tolerated dose
- ECOG :
-
Eastern Cooperative Oncology Group
References
Leyland-Jones B, O'Dwyer P (1986) Biochemical modulation: application of laboratory models to the clinic. Cancer Treat Rep 70:219
Martin D, Stolfi R, Sawyer R, Young C (1985) Application of biochemical modulation with a therapeutically inactive modulating agent in clinical trials of cancer chemotherapy. Cancer Treat Rep 69:421
Darnowsky J, Handschumacher R (1989) Enhancement of fluorouracil therapy by manipulation of tissue uridine pools. Pharmacol Ther 41:381
Ardalan B, Singh G, Silberman H (1988) A randomized phase I and II study of short-term infusion of high-dose fluorouracil with or withoutN-(phosphoacetyl)-L-aspartic acid in patients with advanced pancreatic and colorectal cancers. J Clin Oncol 6:1053
O'Dwyer P, Paul A, Walczak J, Weiner L, Litwin S, Comis R (1990) Phase II study of biochemical modulation of fluorouracil by low-dose PALA in patients with colorectal cancer. J Clin Oncol 8:1497
Dexter D, Hesson D, Ardecky R, Rao G, Tippett D, Dusak B, et al (1985) Activity of a novel 4-quinolinecarboxylic acid: NSC 368390 [6-fluoro-2-(2′-fluor-1,1′-biphenyl-4-yl)-3-methyl-4-quinolinecarboxylic acid sodium salt against experimental tumors. Cancer Res 45:5563
Chen S, Ruben R, Dexter D (1986) Mechanism of action of the novel anticancer agent 6-fluoro-2-(2′-fluor-1,1′-biphenyl-4-yl)-3-methyl-4-quinolinecarboxylic acid sodium salt (NSC 368390): inhibition of de novo pyrimidine nucleotide biosynthesis. Cancer Res 46:5014
Peters G, Sharma S, Laurensse E, Pinedo H (1987) Inhibition of pyrimidine de novo synthesis by DuP 785 (NSC 368390). Invest New Drugs 5:235
Chen S, Perella F, Behrens D, Papp L (1992) Inhibition of dihydroorotate dehydrogenase by brequinar sodium. Cancer Res 52:3521
Peters G, Schwartsmann G, Nadal J, Laurensse E, van Groeningen, C, van der Vijgh W, et al (1990) In vivo inhibition of the pyrimidine de novo enzyme dihydroorotic acid dehydrogenase by brequinar sodium (DUP-785; NSC 368390) in mice and patients. Cancer Res 50:4644
Peters G, Nadal J, Laurensse E, de Kant E, Pinedo H (1990) Retention of in vivo antipyrimidine effects of brequinar sodium (DUP-785; NSC 368390) in murine liver, bone marrow and colon cancer. Biochem Pharmacol 39:135
Moore M, Robert F, Cripps M, Ruckdeschel J, Neidhart J, Natale R, et al (1991) A phase II study of brequinar sodium (DUP 785, NSC 368390) in gastrointestinal (GI) cancers (CA). Proc Am Soc Clin Oncol 10:152
Pizzorno G, Wiegand R, Lentz S, Handschumacher R (1992) Brequinar potentiates 5-fluorouracil antitumor activity in a murine model colon 38 tumor by tissue-specific modulation of uridine nucleotide pools. Cancer Res 52:1160
Bork E, Vest S, Hansen H (1989) A phase I clinical and pharmacokinetic study of brequinar sodium, DUP 785 (NSC 368390), using a weekly and a biweekly schedule. Eur J Clin Oncol 25:1403
Oken M, Creech R, Tormey D, Horton J, Davis TE, McFadden ET, et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649
Arteaga C, Brown T, Kuhn J, Shen H, O'Rourke J, Beougher K, et al (1989) Phase I clinical and pharmacokinetic trial of sodium (DUP 785; NSC 368390). Cancer Res 49:4648
Darnowsky J, Handschumacher R (1985) Tissue specific enhancement of uridine utilization and 5-fluorouracil therapy in mice by benzylacyclouridine. Cancer Res 45:5364
Grem J, King S, O'Dwyer P, Leyland-Jones B (1988) Biochemistry and clinical activity ofN-(phosphonacetyl)-L-aspartate: a review. Cancer Res 48:4441
Martin D, Stolfi R, Sawyer R, Spiegelman S, Casper E, Young C (1983) Therapeutic utility of utilizing low doses ofN-(phosphonacetyl)-L-aspartic acid in combination with 5-fluorouracil: a murine study with clinical relevance. Cancer Res 43:2317
Peters G, Kraal I, Pinedo H (1982) In vitro and in vivo studies on the combination of brequinar sodium (DUP-785; NSC 368390) with 5-fluorouracil; effects of uridine. Br J Cancer 65: 229
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Buzaid, A.C., Pizzorno, G., Marsh, J.C. et al. Biochemical modulation of 5-fluorouracil with brequinar: results of a phase I study. Cancer Chemother. Pharmacol. 36, 373–378 (1995). https://doi.org/10.1007/BF00686185
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00686185