Abstract
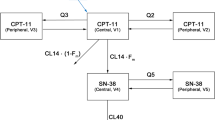
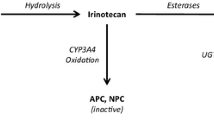
Irinotecan (CPT-11) is a novel topoisomerase I inhibitor with clinical activity in human malignancies. The objective of this study was to develop efficient limited sampling models (LSMs) to estimate simultaneously the area under the plasma concentration versus time curves (AUC) for both CPT-11 and its active metabolite SN-38. A total of 64 pharmacokinetic sets (≥24-h sampling) were obtained in phase I studies at doses ranging from 50 to 750 mg/m2 (0.5-h i.v. infusion). The patients were randomly assigned to a training data set (n=32) and a test set (n=32). Multiple linear regression analyses were used to determine the optimal LSMs based on the correlation coefficient (r), bias (MPE%, percentage of mean prediction error), and precision (RMSE%, percentage of root mean squared prediction error). Of these LSMs, the ones including maximal concentrations of CPT-11 (0.5 h, the end of the i.v. infusion) and metabolite SN-38 (≈ 1 h) were favored along with predictive precision and clinical constraints. Several bivariate models including a 6-h time point as the last sampling time (or 7 h) were found to be highly predictive of either the CPT-11 AUC or the SN-38 AUC. The chosen sampling time points were the ones that allowed the best compromise between the accurate determination of either compound alone with the same sampling times. The simultaneously best prediction of both CPT-11 and SN-38 AUCs was obtained with sampling time points harvested at 0.5, 1, and 6 h (or 7 h). With these sampling time points a trivariate model was selected for the determination of CPT-11 AUC namely, CPT-11 AUC (ng h ml−1)=0.820×C0.5h+0.402×C1h+15.47 ×C6h+928, and a corresponding model was selected for the determination of metabolite AUC, i.e., SN-38 AUC (ng h ml−1)=4.05×C0.5h−0.81×C1h+23.01×C6h−69.78, whereC(t) is the concentration in nanograms per milliliter of either compound at a given timet. These models performed well with the test data sets for CPT-11 AUC (r=0.98, MPE%=−1.4, RMSE%=13.9) and for SN-38 AUC (r=0.95, MPE%=−6.5, RMSE%=37.7). In addition to the determination of AUCs (and hence clearance), these models also allow the determination of the maximal concentrations of both compounds, which might be needed for pharmacodynamics studies. Other bi- and trivariate models including other time points are also presented. These LSMs not only will facilitate ongoing and future clinical trials by significantly reducing the number of blood samples needed for pharmacokinetics studies but will hopefully contribute to a better knowledge of pharmacokinetic-pharmacodynamic relationships for both CPT-11 and its active metabolite SN-38.
Similar content being viewed by others
Abbreviations
- CPT-11 :
-
(7-Ethyl-10-[4-(1-piperidino)-1-piperidino]-carbonyloxy-camptothecin
- SN-38 :
-
7-ethyl-10-hydroxy-camptothecin
- AUC :
-
area under the plasma concentration versus time curve
- MPE% :
-
percentage of mean prediction error (bias)
- RMSE% :
-
percentage root mean squared prediction error (precision)
- MRT :
-
mean residence time
- Vdss :
-
volume of distribution at steady state
- CL :
-
total body clearance
References
Abigerges D, Chabot GG, Armand JP, Hérait P, Gouyette A, Gandia D (1995) Phase I and pharmacologic studies of the camptothecin analogue irinotecan (CPT-11) administered every three weeks in cancer patients. J Clin Oncol 13: 210–221
Barilero I, Gandia D, Armand JP, Mathieu-Boué A, Ré M, Gouyette A, Chabot GG (1992) Simultaneous determination of the camptothecin analogue CPT-11 and its active metabolite SN-38 by high-performance liquid chromatography: application to plasma pharmacokinetic studies in cancer patients. J Chromatogr 575: 275–280
Bissery MC, Mathieu-Boué A, Lavelle F (1991) Preclinical evaluation of CPT-11, a campothecin derivative. Proc Am Assoc Cancer Res 32: 402
Burris HA, Rothenberg ML, Kuhn JG, Von Hoff DD (1992) Clinical trials with the topoisomerase I inhibitors (review). Semin Oncol 19: 663–669
Chabot GG, Forni M de, Abigerges D, Armand JP, Clavel M, Bugat R, Culine S, Extra JM, Marty M, Bissery MC, Mathieu-Boué A, Herait P, and Gouyette A (1995) Clinical trials and pharmacology studies of CPT-11 and its active metabolite SN-38 in France: preliminary pharmacokinetic-pharmacodynamic relationships. In: Potmesil M, Pinedo H (eds) Camptothecins: new anticancer agents. CRC, Boca Raton, Florida pp 83–92
Chabot GG, Abigerges D, Catimel G, Culine S, Forni M de, Extra J-M, Mahjoubi M, Hérait P, Armand J-P, Bugat R, Clavel M, Marty M (1995) Population pharmacokinetics and pharmacodynamics of irinotecan (CPT-11) and active metabolite SN-38 during phase I trials. Ann Oncol 6: 141–151
Chen AY, Yu C, Potmesil M, Wall ME, Wani MC, and Liu LF (1991) Camptothecin overcomes MDR1-mediated resistance in human KB carcinoma cells. Cancer Res 51: 6039–6044
Clavel M, Mathieu-Boué A, Dumortier A, Chabot G.G., Cote C, Bissery M.C., Marty M (1992) Phase I study of CPT-11 administered as a daily infusion for 3 consecutive days (abstract). Proc Am Assoc Cancer Res 33: 262
De Forni M, Bugat R, Chabot GG, Culine S, Extra JM, Gouyette A, Madelaine I, Marty M, Mathieu-Boué A (1994) Phase I and pharmacokinetic study of the camptothecin derivative irinotecan administered on a weekly schedule in cancer patients. Cancer Res 54: 4347–4354
Egorin MJ, Forrest A, Belani CP, Ratain MJ, Abrams JS, Van Echo DA (1989) A limited sampling strategy for cyclosphosphamide pharmacokinetics. Cancer Res 49: 3129–3133
Fukuoka M, Nitani H, Suzuki A, Motomiya M, Hasegawa K, Nishiwaki Y, Kuriyama T, Ariyoshi Y, Negoro S, Masuda N, Nakajima S, Taguchi T, for the CPT-11 Lung Cancer Study Group (1992) A phase II study of CPT-11, a new derivative of camptothecin, for previously untreated non-small cell lung cancer. J Clin Oncol 10: 16–20
Gay C, Lokiec F, Canal P, Bonneterre J, Bugat R, Tubiana-Hulin M, Mathieu-Boué A (1994) Pharmacokinetics and pharmacodynamics of the camptothecin analogue CPT-11 during phase II studies (abstract 1452). Proc Amer Assoc Cancer Res 35: 243
Gibaldi M (1991) Biopharmaceutics and clinical pharmacokinetics, 4th edn. Lea & Febiger, Philadelphia, London
Gupta RS, Gupta R, Eng B, Lock RB, Ross WE, Hertzberg RP, Caranfa MJ, Johnson RK (1988) Camptothecin-resistant mutants of Chinese hamster ovary cells containing a resistant form of topoisomerase I. Cancer Res 48: 6404–6410
Houghton PJ, Cheshire PJ, Hallman JC, Bissery MC, Mathieu-Boué A, Houghton JA (1993) Therapeutic efficacy of the topoisomerase I inhibitor 7-ethyl-10-[4-(1-piperidino)-1-piperidino]-carbonyloxy-camptothecin against human tumor xenografts: lack of cross-resistance in vivo in tumors with acquired resistance to the topoisomerase inhibitor 9-dimethylaminomethyl-10-hydroxycamptothecin. Cancer Res 53: 2823–2829
Hsiang YH, Liu LF (1988) Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res 48: 1722–1726
Hsiang YH, Hertzberg R, Hecht S, Liu LF (1985) Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem 260: 14873–14878
Hsiang YH, Liu LF, Wall ME, Wani MC, Nicholas AW, Maikumar, G, Kirschenbaum S, Silber R, Potmesil M (1989) DNA topoisomerase I mediated DNA cleavage and cytotoxicity of camptothecin analogues. Cancer Res 49: 4835–4839
Kanzawa F, Sugimoto Y, Minato K, Kasahara K, Bungo M, Nakagawa K, Fujiwara Y, Liu F, Saijo N (1990) Establishment of a camptothecin analogue (CPT-11)-resistant cell line of human small cell lung cancer: characterization and mechanism of resistance. Cancer Res 50: 5919–5924
Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K (1991) Intracellular roles of SN-38, a metabolite of the camptothecin derivative, CPT-11, in the antitumor effect of CPT-11. Cancer Res 51: 4187–4191
Kessel D, Bosmann HB, Lohr K (1972) Camptothecin effects on DNA synthesis in murine leukemia cells. Biochim Biophys Acta 269: 210–216
Kunimoto T, Nitta K, Tanaka T, Uehara N, Baba H, Takeuchi M, Yokokura T, Sawada S, Miyasaka T, Mutai M (1987) Antitumor activity of 7-ethyl-10-[4-(1-piperidino)-1-piperidino]-carbonyloxy-camptothecin, a novel water-soluble derivative of camptothecin, against murine tumors. Cancer Res 47: 5944–5947
Liu LF (1989) DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem 58: 351–375
Madelaine I, Prost S, Naudin A, Riou G, Lavelle F, Riou JF (1993) Sequential modifications of topoisomerase I activity in a camptothecin-resistant cell line established by progressive adaptation. Biochem Pharmacol 45: 339–348
Masuda N, Fukuoka M, Kusunoki Y, Matsui K, Takifuji N, Kudoh S, Negoro S, Nishioka M, Nakagawa K, Takada M (1992) CPT-11: a new derivative of camptothecin for the treatment of refractory or relapsed small-cell lung cancer. J Clin Oncol 10: 1225–1229
Matsuzaki T, Yokokura T, Mutai M, Tsuruo T (1988) Inhibition of spontaneous and experimental metastasis by a new derivative of camptothecin, CPT-11, in mice. Cancer Chemother Pharmacol 21: 308–312
Negoro S, Fukuoka M, Masuda N, Takada M, Kusunoki Y, Matsui K, Takifuji N, Kudoh S, Niitani H, Taguchi T (1991) Phase I study of weekly intravenous infusion of CPT-11, a new derivative of camptothecin, in the treatment of advanced nonsmall cell lung cancer. J Natl Cancer Inst 83: 1164–1168
Ohe Y, Sasaki Y, Shinkai T, Eguchi K, Tamura T, Kojima A, Kunikane H, Okamoto H, Karato A, Ohmatsu H, Kanzawa F, Saijo N (1992) Phase I study and pharmacokinetics of CPT-11 with 5-day continuous infusion. J Natl Cancer Inst 84: 972–974
Ohno R, Okada K, Masaoka T, Kuramoto A, Arima T, Yoshida Y, Ariyoshi H, Ichimaru M, Sakai Y, Oguro M, Ito Y, Morishima Y, Yokomaku S, Ota K (1990) An early phase II study of CPT-11: a new derivative of camptothecin for the treatment of leukemia and lymphoma. J Clin Oncol 8: 1907–1912
Ratain MJ, Vogelzang NJ (1987) Limited sampling model for vinblastine pharmacokinetics. Cancer Treat Rep 71: 935–939
Ratain MJ, Staubus AE, Schilsky RL, Malspeis L (1988) Limited sampling models for amonafide (NSC 308847) pharmacokinetics. Cancer Res 48: 4127–4130
Rothenberg ML, Kuhn JG, Burris HA III, Nelson J, Eckardt JR, Tristan-Morales M, Hilsenbeck SG, Weiss GR, Smith LS, Rodriguez GI, Rock MK, Von Hoff DD (1993) Phase I and pharmacokinetic trial of weekly CPT-11. J Clin Oncol 11: 2194–2204
Rowinsky EK, Grochow LB, Ettinger DS, Sartorius SE, Lubejko BG, Chen T-L, Rock MK, Donehower RC (1994) Phase I and pharmacological study of the novel topoisomerase I inhibitor 7-ethyl-10-[4-(1-piperidino)-1-piperidino]-carbonyloxycamptothecin (CPT-11) administered as a ninety-minute infusion every 3 weeks. Cancer Res 54: 427–436
Sheiner LB, Beal SL (1981) Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 9: 503–512
Slichenmyer WJ, Rowinsky EK, Donehower RC, Kaufmann SH (1993) The current status of camptothecin analogues as antitumor agents (review). J Natl Cancer Inst 85: 271–291
Sugimoto Y, Tsukahara S, Oh-Hara T, Isoe T, Tsuruo T (1990) Decreased expression of DNA topoisomerase I in camptothecin-resistant tumor cell lines as determined by a monoclonal antibody. Cancer Res 50: 6925–6930
Taguchi T, Wakui A, Hasegawa K (1990) Phase I clinical study of CPT-11. Research Group of CPT-11. Jpn J Cancer Chemother 17: 115–120
Takeda S, Shimazoe T, Kuga H, Sato K, Kono A (1992) Camptothecin analog (CPT-11)-sensitive human pancreatic tumor cell line QGP-1N shows resistance to SN-38, an active metabolite of CPT-11. Biochem Biophys Res Commun 188: 70–77
Tamura H, Kohchi C, Yamada R (1991) Molecular cloning of a cDNA of a camptothecin-resistant human DNA topoisomerase I and identification of mutation sites. Nucleic Acids Res 51: 1129–1136
Tsuruo T, Matsuzaki T, Matsushita M, Saito H, Yokokura T (1988) Antitumor effect of CPT-11, a new derivative of camptothecin, against pleiotropic drug-resistant tumors in vitro and in vivo. Cancer Chemother Pharmacol 21: 71–74
Van Warmerdam LJC, Verweij J, Rosing H, Schellens JHM, Maes RAA, Beijnen JH (1994) Limited sampling models for topotecan pharmacokinetics. Ann Oncol 5: 259–264
Wang JC (1985) DNA topoisomerases. Annu Rev Biochem 54: 665–697
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chabot, G.G. Limited sampling models for simultaneous estimation of the pharmacokinetics of irinotecan and its active metabolite SN-38. Cancer Chemother. Pharmacol. 36, 463–472 (1995). https://doi.org/10.1007/BF00685795
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00685795