Abstract
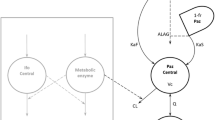
Docetaxel, a novel anticancer agent, was given to 26 patients by short i.v. infusion (1–2 h) at various dose levels (70–115 mg/m2, the maximum tolerated dose) during 2 phase I studies. Two population analyses, one using NONMEM (nonlinear mixed-effect modeling) and the other using NPML (nonparametric maximum-likelihood), were performed sequentially to determine the structural model; estimate the mean population parameters, including clearance (Cl) and interindividual variability; and find influences of demographic covariates on them. Nine covariates were included in the analyses: age, height, weight, body surface area, sex, performance status, presence of liver metastasis, dose level, and type of formulation. A three-compartment model gave the best fit to the data, and the final NONMEM regression model for Cl wasCl=BSA(θ1+θ2×AGE), expressing Cl (in liters per hour) directly as a function of body surface area. Only these two covariates were considered in the NPML analysis to confirm the results found by NONMEM. Using NONMEM [for a patient with mean AGE (52.3 years) and mean BSA (1.68 m2)] and NPML, docetaxel Cl was estimated to be 35.6 l/h (21.2 lh−1 m−2) and 37.2 l/h with interpatient coefficients of variation (CVs) of 17.4% and 24.8%, respectively. The intraindividual CV was estimated at 23.8% by NONMEM; the corresponding variability was fixed in NPML in an additive Gaussian variance error model with a 20% CV. Discrepancies were found in the mean volume at steady state (Vss; 83.2 l for NPML versus 124 l for NONMEM) and in terminal half-lives, notably the meant 1/2γ, which was shorter as determined by NPML (7.89 versus 12.2 h), although the interindividual CV was 89.1% and 62.7% for Vss andt 1/2γ, respectively. However, the NPML-estimated probability density function (pdf) oft 1/2γ, was bimodal (5 and 11.4 h), probably due to the imbalance of the data. Both analyses suggest a similar magnitude of mean Cl decrease with small BSA and advanced age.
Similar content being viewed by others
References
Aapro MS, Zulian G, Alberto P, Bruno R, Oulid-Aissa D, Le Bail N (1992) Phase I and pharmacokinetic study of RP 56976 in a new ethanol-free formulation of Taxotere (abstract 208). Ann Oncol 3 [Suppl 5]:53
Beal SL, Boeckmann AJ, Sheiner LB (1988–1990) NONMEM user's guide, parts I–VI. University of California at San Francisco, San Francisco, California
Bruno R, Sanderink G (1993) Pharmacokinetics and metabolism of Taxotere (docetaxel). In: Workman P, Graham MA (eds) Pharmacokinetics and cancer chemotherapy. (Imperial Cancer Research Fund Cancer Surveys, vol 17). Cold Spring Harbor Laboratory Press, New York, pp 305–313
Bruno R, Iliadis MC, Lacarelle B, Cosson V, Mandema JW, Le Roux Y, Montay G, Durand A, Ballereau M, Alasia M, Albanese J, Francois G, Iliadis A, Frydman A (1992) Evaluation of Bayesian estimation in comparison to NONMEM for population pharmacokinetic data analysis: application to pefloxacin in intensive care unit patients. J Pharmacokinet Biopharm 6: 653–669
Bruno R, Vergniol JC, Montay G, et al (1992) Clinical pharmacology of Taxotere (RP 56976) given as 1–2 hour infusion every 2–3 weeks. Proc Am Assoc Cancer Res 33:261
Bruno R, Cosson V, Vergniol JC, Montay G, Le Bail N, Bayssas M, Marty M, Clavel M, Aapro M, Alberto P, Frydman A (1993) Taxotere population pharmacokinetics (abstract 1396). Proc Am Assoc Cancer Res 34:234
Bruno R, Dorr MB, Montay G, Frydman A, This F, Fumoleau P, Kay S, Kavanagh GH, Burris HA, Rigas JR, Bayssas M (1994) Design and prospective implementation of population pharmacokinetic studies during the development of docetaxel (RP 56976), a new anticancer drug (abstract PII-35). Clin Pharmacol Ther 55:161
Extra JM, Rousseau F, Bruno R, Clavel M, Le Bail N, Marty M (1993) Phase I and pharmacokinetic study of Taxotere (RP 56976, NSC 628503) given as short intravenous infusion. Cancer Res 53:1037–1042
Jochemsen R (1992) Current experience of population pharmacokinetics within the pharmaceutical industry: an introduction. In: Rowland M, Aarons L (eds) New strategies in drug development and clinical evaluation: the population approach. Commission of European Communities, Brussels, pp 31–40
Launay-Iliadis MC, Bruno R, Montay G, Frydman A, Marty M, Clavel M, Aapro M, Iliadis A (1993) Population pharmacokinetics of Taxotere using non-parametric maximum likelihood (NPML) software. Fifth European Congress of Biopharmaceutics and Pharmacokinetics (abstract 365). Eur J Drug Metab Pharmacokinet [Suppl] 18:193
Lejeune M (1984) Estimation non-paramétrique par noyaux: régression polynômiale mobile. Rev Stat Appl 23:43–67
Mallet A (1986) A maximum likelihood estimation method for random coefficient regression models. Biometrika 73: 645–656
Mallet A (1989) Introduction to NPML version 1. Service d'Informatique Médicale, INSERM U194, Paris
Mantré F, Mallet A (1992) Experiences with NPML — application to dosage individualization of cyclosporine, gentamicin and zidovudine. In: Rowland M, Aarons L (eds) New strategies in drug development and clinical evaluation: the population approach, Commission of European Communities, Brussels, pp 75–90
Sambol NC (1992) The population approach: applications to experimental data. In: Rowland M, Aarons L (eds) New strategies in drug development and clinical evaluation: the population approach. Commission of European Communities, Brussels, pp 183–191
Sheiner LB, Grasela TH (1991) An introduction to mixed effect modeling: concepts, definitions and justification. J Pharmacokinet Biopharm 19 [Suppl] 11s-24s
Sheiner LB, Ludden TM (1992) Population pharmacokinetics/dynamics. Annu Rev Pharmacol Toxicol 32:185–209
Sheiner LB, Rosenberg B, Marathe VV (1977) Estimation of population characteristics of pharmacokinetic parameters from routine clinical data. J Pharmacokinet Biopharm 5: 445–479
Vergniol JC, Bruno R, Montay G, Frydman A (1992) Determination of Taxotere in human plasma by a semi-automated high-performance liquid chromatographic method. J Chromatogr 582:273–278
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Launay-Iliadis, M.C., Bruno, R., Cosson, V. et al. Population pharmacokinetics of docetaxel during phase I studies using nonlinear mixed-effect modeling and nonparametric maximum-likelihood estimation. Cancer Chemother. Pharmacol. 37, 47–54 (1995). https://doi.org/10.1007/BF00685628
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00685628