Abstract
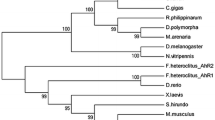
We have isolated a cDNA clone of mRNA for the cytochrome P450 from a 3-methylcholanthrene (MC)-treated red sea bream,Pagrus major, using a cDNA fragment for rat P4501A2 as a probe. The cloned cDNA is ca. 1.8 kb long and contains an open reading frame of 1545 nucleotides for polypeptides of 515 amino acids. The deduced N-terminal amino acid sequence of the cDNA is very similar to that for purified cytochrome P450 protein from the marine fish scup, which was reported previously (Klotz et al. 1983). A conserved amino acid sequence containing a putative heme-binding cysteine is present in the equivalent position proximate to the C-terminus of the molecules. The deduced amino acid sequence shows more than 50% positional identity with known members of the mammalian aromatic hydrocarbon-inducible P450 family. RNA blot analysis indicates that P450 mRNA (s) is expressed in the liver, kidney, gill and gut of the MC-treatedP. major.
Similar content being viewed by others
References
Besten PJ, Herwig HJ, Donselaar EG, Livingstone DR (1990) Cytochrome P450 monooxygenase system and benzo (a) pyrene metabolism in echinoderms. Mar Biol 107:171–177
Chaplin DD, Galbraith LJ, Seidman JG, White PC, Parker KL (1986) Nucleotide sequence analysis of murine 21-hydroxylase genes: mutations affecting gene expression. Proc natn Acad Sci USA 83:9601–9605
Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ (1979) Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry, Am chem Soc Easton, PA 18:5294–5299
Cooper KR, Prince R, Haasch ML, Wejksnora PJ, Lech JJ (1990) Detection of cytochrome P450 induction in feral and caged fish. Toxicologist 10:249
Feyereisen R, Koener JF, Farnsworth DE, Nebert DW (1989) Isolation and sequence of cDNA encoding a cytochrome P450 from an insecticide-resistant strain of the house fly,Musca domestica. Proc natn Acad Sci USA 86:1465–1469
Fujii-Kuriyama Y, Mizukami Y, Kawajiri K, Sogawa K, Muramatsu M (1982) Primary structure of a cytochrome P450: coding nucleotide sequence of phenobarbital-inducible cytochrome P450 cDNA from rat liver. Proc natn Acad Sci USA 79:2793–2797
Fujii-Kuriyama Y, Taniguchi T, Mizukami Y, Sakai M, Tashiro Y, Muramatsu M (1981) Construction and identification of a hybrid plasmid containing DNA sequence complementary to phenobarbital-inducible cytochrome P450 messenger RNA from rat liver. J Biochem 89:1869–1879
Gabriac B, Werck-Reichhart D, Teutsch H, Durst F (1991) Purification and immunocharacterization of a plant cytochrome P450: the cinnamic acid 4-hydroxylase. Archs Biochem Biophys 288: 302–309
Gassman NJ, Kennedy CJ (1992) Cytochrome P450 content and xenobiotic metabolizing enzyme activities in the scleractinian coral,Favia fragum. Bull mar Sci 50:320–330
Goksøyr A, Förlin L (1992) The cytochrome P450 system in fish, aquatic toxicology and environmental monitoring. Aquat Toxic 22:287–311
Gonzalez FJ, Kimura S, Nebert DW (1985) Comparison of the flanking regions and introns of the mouse 2,3,7,8-tetrachlorodibenzo-p-dioxin-inducible cytochrome P1450 and P3450 genes. J biol Chem 260:5040–5049
Gooch JW, Elskus AA, Kloepper-Sams PJ, Hahn ME, Stegeman JJ (1989) Effects ofortho and non-ortho substituted polychlorinated biphenyl congeners on the hepatic monooxygenase system in scup (Stenotomus chrysops). Toxicol appl Pharmac 98:61–65
Gotoh H, Tagashira Y, Iizuka T, Fujii-Kuriyama Y (1983) Structural characteristics of cytochrome P450. Possible location of the heme-binding cysteine in determined amino-acid sequences. J Biochem 93:807–817
Grunstein M, Hogness DS (1975) Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc natn Acad Sci USA 72:3961–3965
Gubler U, Hoffman BJ (1983) A simple and very efficient method for generating cDNA libraries. Gene (Amsterdam) 25:263–269
Haasch ML, Quardokus EM, Sutherland LA, Goodrich MS, Prince R, Cooper KR, Lech JJ (1992) CYP1A1 protein andmRNA in teleosts as an environmental bioindicator: laboratory and environmental studies. Mar envirl Res 34:139–145
Haniu M, Armes LG, Tanaka M, Yasunobu TK, Wagner GC, Gunsalus IC (1982) The primary structure of the monoxygenase cytochrome P450cam. Biochem biophys Res Commun 105:889–894
Hansson T Rafter J, Gustafsson J-A (1980) Effects of some common inducers on the hepatic microsomal metabolism of androstenedione in rainbow trout with special reference to cytochrome P450 dependent enzymes. Biochem Pharmac 29:581–587
Kawajiri K, Gotoh O, Sogawa K, Tagashira Y, Muramatsu M, Fujii-Kuriyama Y (1984a) Coding nucleotide sequence of 3-methylcholanthrene-inducible cytochrome P450 cDNA from rat liver. Proc natn Acad Sci USA 81:1649–1653
Kawajiri K, Gotoh O, Tagashira Y, Sogawa K, Fujii-Kuriyama Y (1984b) Titration of mRNAs for cytochrome P450c and P450d under drug-inductive conditions in rat liver by their specific probes of cloned DNAs. J biol Chem 259:10145–10149
Kloepper-Sams PJ, Park SS, Gelboin HV, Stegeman JJ (1987) Specificity and cross-reactivity of monoclonal and polyclonal antibodies against cytochrome P450E of the marine fish scup. Archs Biochem Biophys 253:268–278
Klotz AV, Stegeman JJ, Walsh C (1983) An aryl hydrocarbon hydroxylating hepatic cytochrome P450 from the marine fishStenotomus chrysops. Archs Biochem Biophys 226:578–592
Klotz AV, Stegeman JJ, Woodin BR, Snowberger EA, Thomas PE, Walsh C (1986) Cytochrome P450 isozymes from marine teleostStenotomus chrysops: their roles in steroid hydroxylation and the influence of cytochromeb 5. Archs Biochem Biophys 249:326–338
Kreamer G-L, Squibb K, Gioeli D, Garte SJ, Wirgin I (1991) Cytochrome P4501A mRNA expression in feral Hudson River tomcod. Envir Res 55:64–78
Lee RF, Singer SC, Page DS (1981) Responses of cytochrome P450 systems in marine crab and polychaetes to organic pollutants. Aquat Toxic 1:355–365
Lorenzana RM, Hedstrom OR, Buhler DR (1988) Localization of cytochrome P450 in the head and trunk kidney of rainbow trout (Salmo gairdneri). Toxicol appl Pharmac 96:159–167
Lu AYH, West SB (1980) Multiplicity of mammalian microsomal cytochrome P450. Pharmac Rev 31:277–295
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Maxam AM, Gilbert W (1977) A new method for sequencing DNA. Proc natn Acad Sci USA 74:560–564
Melancon MJ, Elcombe CR, Vodicnir MJ, Lech JJ (1981) Induction of cytochrome P450 and MFO activity by polychlorinated biphenyls and β-naphtoflavone in the carp (Cyprinus carpio). Comp Biochem Physiol 69C:219–226
Miller MR, Hinton E, Blair JJ, Stegeman JJ (1988) Immunohistochemical localization of cytochrome P450E in liver, gill and heart of scup (Stenotomus chrysops) and rainbow trout (Salmo gairdneri). Mar envirl Res 24:37–39
Miller MR, Hinton DE, Stegeman JJ (1989) Cytochrome P450E induction and localization in gill pillar (endothelial) cells of scup and rainbow trout. Aquat Toxic 14:307–322
Mizukami Y, Sogawa K, Muramatsu M, Fujii-Kuriyama Y (1983) Gene structure of a phenobarbital-inducible cytochrome P450 in rat liver. Proc natn Acad Sci USA 80:3958–3962
Nebert DW, Nelson DR, Adesnik M, Coon MJ, Estabrook RW, Gonzalez FJ, Guengerich FP, Gunsalus IC, Jonson EF, Kemper B, Levin W, Philips IR, Sato R, Waterman MR (1989) The P450 superfamily: updated listing of all genes and recommended nomenclature for the chromosomal loci. DNA 8:1–13
Park SS, Miller H, Klotz AV, Kloepper-Sams PJ, Stegeman JJ, Gelboin HV (1986) Monoclonal antibodies to liver microsomal cytochrome P450E of the marine fishStenotomus chrysops (scup): cross reactivity with 3-methylcholanthrene induced rat cytochrome P450. Archs Biochem Biophys 249:339–350
Payne JF, Fancey LL, Rahimtula AD, Porter EL (1987) Review and perspective on the use of mixed-function oxygenase enzymes in biological monitoring. Comp Biochem Physiol 86C:233–245
Peacock SL, McIver CM, Monohan JJ (1981) Transformation ofE. coli using homopolymer-linked plasmid chimeras. Biochim Biophys Acta 655:243–251
Ryan DE, Thomas PE, Korzeniowsky D, Levin W (1979) Separation and characterization of highly purified forms of liver-microsomal cytochrome P450 from rats treated with polychlorinated biphenyls, phenobarbital and 3-methylcholanthrene. J biol Chem 254:1365–1374
Sanger F, Coulson AR, Barrell BG, Smith AJH, Roe BA (1980) Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J molec Biol 143:161–178
Sanglard D, Kappeli O, Fiechter A (1984) Metabolic conditions determining the composition and catalytic activity of cytochrome P450 monooxygenase inCandida tropicalis. J Bact 157:297–302
Sato R, Omura T (1978) Cytochrome P450. Kodansha, Tokyo
Singer SC, March PE, Gonsoulin F, Lee RF (1980) Mixed function oxygenase activity in the blue crab,Callinectes sapidus: characterization of enzyme activity from stomach tissue. Comp Biochem Physiol 65:129–134
Smolowitz RM, Hahn ME, Stegeman JJ (1991) Immunohistochemical localization of cytochrome P450 IA1 induced by 3,3′,4,4′-tetrachloro-biphenyl and by 2,3,7,8-tetrachlorodibenzoa-furan in liver and extrahepatic tissues of the teleostStenotomus chrysops (Scup). Drug Metab Disposition 19:113–123
Sogawa K, Gotoh O, Kawajiri K, Fujii-Kuriyama Y (1984) Distinct organization of methylcholanthrene- and phenobarbital-inducible cytochrome P450 gene in the rat. Proc natn Acad Sci USA 81:5066–5070
Stegeman JJ, Kloepper-Sams PJ (1987) Cytochrome P450 isozymes and monooxygenase activity in aquatic animals. Envir Hlth Perspectives 71:87–95
Stegeman JJ, Klotz AV, Woodin BR, Pajor AM (1981) Induction of hepatic cytochrome P450 in fish and the indication of environmental induction in scup (Stenotomus chrysops). Aquat Toxic 1:197–212
Stegeman JJ, Teng FY, Snowberger EA (1987) Induced cytochrome P450 in winter flounder (Pseudopleuronectes americanus) from coastal Massachusetts evaluated by catalytic assay and monoclonal antibody probes. Can J Fish aquat Sciences 44:1270–1277
Stegeman JJ, Woodin B, Park S, Kloepper-Sams P, Gelboin H (1985) Microsomal cytochrome P450 function in fish evaluated with polyclonal and monoclonal antibodies to cytochrome P450E from scup. Mar envirl Res 16:83–86
Tarr GE, Black SD, Fujita VS, Coon MJ (1983) Complete amino acid sequence and predicted membrane topology of phenobarbital-induced cytochrome P450 (isozyme 2) from rabbit liver microsomes. Proc natn Acad Sci USA 80:6552–6556
Teunissen Y, Geraerts WPM, Heerikhuizen HV, Planta RJ, Joosse J (1992) Molecular cloning of a cDNA encoding a member of a novel cytochrome P450 family in the molluscLymnaea stagnalis. J Biochem 112:249–252
Van Veld PA, Westbrook DJ, Woddin BR, Hale RC, Smith CL, Huggett RJ, Stegeman JJ (1990) Induced cytochrome P450 in intestine and liver of spot (Leiostomus xanthurus) from a polycyclic aromatic hydrocarbon contaminated environment. Aquat Toxic 17:119–132
Williams DE, Buhler DR (1982) Purification of cytochrome P448 from β-naphthoflavone treated rainbow trout. Biochem Biophys Acta 717:398–404
Williams DE, Buhler DR (1984) Benzo[a]pyrene-hydroxylase catalyzed by purified isozymes of cytochrome P450 from β-naphthoflavone-fed rainbow trout. Biochem Pharmac 33:3743–3753
Yoshioka H, Morohashi K, Sogawa K, Yamane M, Kominami S, Takemori S, Okada Y, Omura T, Fujii-Kuriyama Y (1986) Structural analysis of cloned cDNA for mRNA of microsomal cytochrome P450 (c21) which catalyze steroid 21-hydroxylation in bovine adrenal cortex. J biol Chem 261:4106–4109
Zuber MX, John ME, Okamura T, Simpson ER, Waterman MR (1986) Bovine adrenocortical cytochrome P45017 α. J biol Chem 261:2475–2482
Author information
Authors and Affiliations
Additional information
Communicated by T. Ikeda, Nagasaki
Rights and permissions
About this article
Cite this article
Mizukami, Y., Okauchi, M., Arizono, K. et al. Isolation and sequence of cDNA encoding a 3-methylcholanthrene-inducible cytochrome P450 from wild red sea bream,Pagrus major . Mar. Biol. 120, 343–349 (1994). https://doi.org/10.1007/BF00680207
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00680207