Abstract
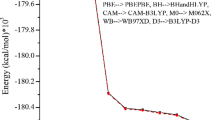
The structures of several naphthalene derivatives and their35Cl NQR spectra have been investigated. 1,8-Diaminonaphthalene,C 92v -Pna2 1, Z = 8,a (in pm) = 881,b = 1577,c = 1213; 1,8-diaminonaphthalene monodichloroacetate,C 62h -C2/c, Z = 8,a = 2050,b = 584,c = 2333,β (in degrees) = 110.1; 1,8-diaminonaphthalene monotrichloroacetate,C 11 -P¯1, Z=2,a=1211,b=1062,c=589,α=74.8,β=80.1,γ=70.9; 1-aminonaphthalene trichloroacetate,D 152h -Pbca, Z=8,a=2347,b=1289,c=889. The35Cl NQR spectrum of 1,8-diaminonaphthalene monodichloroacetate is a doublet, the frequencies decreasing with increasing temperature from 77 to 217 K at which temperatureT b the NQR signals bleach out. A35Cl NQR triplet is found for 1,8-diaminonaphthalene monotrichloracetate in the range 77 ≤ 77K ≤ 207 (=T b ). 1-Amino-naphthalene trichloroacetate shows a35Cl NQR triplet, too, withT b = 136 K. Characteristic for the intermolecular interactions are hydrogen bonds in the chloroacetic acid salts; each NH3 group forms three hydrogen bonds, and of the two oxygens one is involved in two such bonds, one in one bond. Thereby units of two cations and two anions are formed, and these dirners are connected to strings by hydrogen bonds. Additional van der Waals interactions between the chlorine atoms and the naphthalene ring system are observed. Comparison of the intramolecular bond distances C(i)-C(j) of the C10 naphthalene skeleton for 41 naphthalene derivatives (present data and literature) shows that the bond distances C(i)-C(j)are little influenced by substitution, as is the mean bond length. Shorter and longer distances prove a partial localization of charge at C(1)-C(2), C(3)-C(4), C(5)-C(6), and C(7)-C(8). Regularities within the bond angles and characteristic influences of 1,8-disubstitution on it are discussed.
Similar content being viewed by others
References
Biedenkapp, D.; Weiss, Al.Ber. Bunsenges. Phys. Chem. 1966,70, 788.
Fichtner, W.; Marckworth, A.; Weiden, N.; Weiss, Al. Z.Naturforsch.,1986,41a, 215.
Marckworth, A.; Weiden, N.; Weiss, Al.Ber Bunsenges Phys. Chem. 1987,91, 1158.
Basaran, R.; Dou, S.-Q.; Weiss, Al.Ber Bunsenges. Phys. Chem. 1991,95, 46.
Basaran, R.; Dou, S.-q.; Weiss, Al. Z.Naturforsch,1992,47a, 241.
Marckworth, A.; Paulus, H.; Weiden, N.; Weiss, Al. ZPhys. Chem. (Frankfurt) 1991,173, 1.
Basaran, R.; Dou, S.-q.; Weiss, Al.Ber Bunsenges. Phys. Chem. 1992,96, 35.
de Aguiar, A.Ber. Deutsch. Chem. Ges. 1870,3, 27. Ekstrand, A. G.J. Prakt. Chem. 1888,38, 241.
Sheldrick, G. M. SHELXS86, Program for crystal structure solution, University of Göttingen, Germany, 1986.
Sheldrick, G. M. SHELX76, Program for crystal structure determination. University of Cambridge, England, 1976.
Bayer, H.Z Phys. 1951,130, 227.
Krygowski, T. M. InProgress in Physics and Organic Chemistry; Taft, R. W. Ed.1990,17, 239.
Bühl, M.; Steinke, T.; von Raqué Schleyer, P.; Boese, R..Angew. Chem. 1991,103, 1179.
Einspahr, H.; Robert, J.-B; Marsh, R. E.; Roberts, J. D.Acta Crystallogr.,1973,29, 1611.
Herbstein, F. H.Acta Crystallogr., Sect. B 1979,35, 1661.
Sim, G. A.Acta Crystallogr., Sect. B 1982,38, 623.
Evrad, G.; Piret, P.; van Meersche, M.Acta Crystallogr., Sect. B 1972,28, 497.
Cruickshank, D. W. J.,Acta Crystallogr., Sect. B 1957,10, 504.
Pawley, G. S.; Yeats, E. A.Acta Crystallogr., Sect. B 1969,25, 2009.
Wong-Ng, W.; Nyburg, S. C., Awwal, A.; Jankie, R.; Kresge, A.J. Acta Crystallogr., Sect. B 1982,38, 559.
Robert, J.-B.; Sherfinski, J. S.; Marsh, R. E.; Roberts, J. D.J. Org. Chem. 1974,39, 1152.
Banerjee, A.; Brown, C.J. Acta Crystallogr., Sect. C 1985,41, 82.
Foss, L. I.; Syed, A.; Stevens, E. D.; Klein, C. L.Acta Crystallogr, Sect. C 1984,40, 272.
Bush, B. F.; Lynch, V. M.; Lagowski, J.J. Organometallics 1987,6, 1267, Kündig, E. P.; Perret, C; Spichiger, S.; Bernadinelli, G.J. Organomet. Chem. 1985,286, 183.
Meresse, A.; Courseille, C.; Leroy, F.; Chanh, N. B.Acta Crystallogr., Sect. B 1975,31, 1236.
Bright, D.; Maxwell, I. E.; de Boer, J.J. Chem. Soc., Perkin Trans. 2 1973, 2101.
Sooriyakumaran, R.; Boudjouk, P.; Garvey, R. G.Acta Crystallogr., Sect. C 1985,41, 1348.
Blount, J. F.; Cozzi, F.; Damewood, Jr., J. R.; Iroff, L. D.; Sjöstrand, U.; Mislow, K.J. Am. Chem. Soc. 1980,102, 99.
Schweizer, W. B.; Procter, G.; Kaftory, M.; Dunitz, J. D.Helv. Chim. Acta 1978,61, 2783.
Jameson, M. B.; Penfold, B. R.J. Chem. Soc. (London) 1965, 528.
Gafner, G.; Herbstein, F. H.Acta Crystallogr.,1962,15, 1081.
Born, L.; Heywang, G. Z.Kristallogr. 1990,190, 147.
Nagawa, Y.; Goto, M.; Honda, K.; Nakanishi, H.Bull. Chem. Soc. Jpn. 1988,61, 3553.
Handal, J.; White, J. G.; Franck, R. W.; Yuh, Y. H.; Allinger, N. L.J. Am. Chem. Soc. 1977,99, 3345.
Rajan, S. S.Acta Crystallogr., Sect. B 1978,34, 998.
Yagi, E.; Ohashi, Y.; Ishige, M.; Maeda, K.Acta Crystallogr., Sect. C 1989,45, 1109.
Kuroda, R.; Mason, S. F.J. Chem. Soc. Perkin II 1981, 167.
Gali, S.; Solans, X; Miravitlles, C; Font-Altaba, M.Acta Crystallogr., Sect. B 1978,34, 1011.
Fachinformationszentrum Karlsruhe, Gesellschaft für wissenschaftlich-technische Information mbH, D-76344 Eggenstein-Leopoldshafen 2, Germany.
Wigand, S.; Weiden, N.; Weiss, Al.Z Naturforsch., A 1990,45, 490.
Pratt-Brock, C. Dunitz, J. D.Acta Crystallogr., Sect. B 1982,38, 2218.
Cox, E. G.Rev. Mod. Phys. 1958,30, 159.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Basaran, R., Dou, Sq. & Weiss, A. Bond distances and bond angles of the C10 skeleton in naphthalene derivatives as hard bond parameters:35Cl NQR and structure of 1,8-diaminonaphthalene · Cl2HCCOOH, 1,8-diaminonaphthalene · Cl3CCOOH, 1-aminonaphthalene · Cl3CCOOH, and 1,8-diaminonaphthalene. Struct Chem 4, 219–233 (1993). https://doi.org/10.1007/BF00673697
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00673697