Abstract
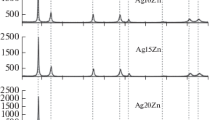
Lattice parameters of Ag-Mg (0.5 and 1 at.%) were measured. A comparison between these results and data previously obtained by gravimetry shows that (a) a high dilatation is due mainly to excess oxygen by comparison with the amount necessary to form stoichiometric MgO oxide, and (b) the earliest stage of Mg oxidation occurs in a non-expanded layer beneath the expanded one, with the formation of substoichiometric species (O/Mg < 1). The formation of substoichiometric species is explained by taking account of strain fields close to O and Mg atoms in the silver lattice. Indeed, the strong deformation introduced by oxygen in interstitial position delays the addition of oxygen on the MgO* and Mg2O* initial species and favors the formation of substoichiometric species.
Similar content being viewed by others
References
J. L. Meijering and M. J. Druyvesteyn,Philips. Res. Rep. 2, 81 (1947).
M. J. Klein and R. A. Huggins,Acta Metall. 10, 55 (1962).
A. Combe, L. Charrin, G. Moya, and J. Cabane,Acta. Metall. 31, 1019 (1983).
L. Charrin, A. Combe, and G. Moya,J. Therm. Anal. 14, 89 (1978).
L. Charrin, A. Combe, and G. Moya,Acta. Metall. 29, 1593 (1981).
S. Guruswamy, S. M. Park, J. P. Hirth, and R. A. Rapp,Oxid. Met. 26, 77 (1986).
L. D. Pethe, H. B. Mathur, and A. B. Biswass,Can. J. Chem. 46, 1187 (1968).
A. Combe and J. Cabane,Oxid. Met. 21, 21 (1984).
L. Charrin, A. Combe, and J. Cabane,Oxid. Met. 37, 65 (1992).
B. M. Semega, L. Charrin, A. Combe, and J. Aride,Phil. Mag. A 66, 1139 (1992).
A. Charai, A. Combe, C. Boulesteix, and J. Cabane,Scripta. Metall. 17, 833 (1983).
A. Charai and G. Nihoul,Phil. Mag. A 58, 571 (1988).
C. Wagner,Z. Elektrochem. 63, 772 (1959).
L. S. Darken,Trans. Am. Soc. Met. 54, 600 (1961).
J. S. Hirschhorn,Met. Sci. J. 1, 91 (1967).
J. L. Meijering,Adv. Mater. Res. 5, 1 (1971).
J. S. Hirschhorn and F. V. Lenel,Trans. Am. Soc. Met. 59, 208 (1966).
J. L. Meijering and M. J. Druyvesteyn,Philips. Res. Rep. 2, 81 (1947).
H. H. Podgurski and F. N. Davis,Trans. Met. Soc. AIME 230, 731 (1964).
J. L. Meijering,Trans. Met. Soc. AIME 218, 968 (1960).
P. R. Swann, S. Weissmann, and D. F. Wriedt,Trans. Met. Soc. AIME 230, 1306 (1964).
H. A. Wriedt,Trans. Met. Soc. AIME 221, 377 (1961).
L. Charrin, A. Combe, F. Cabane, and J. Cabane (to be published).
L. Charrin, Thesis, Marseille, 1987.
A. Krawitz and R. Sinclair,Phil. Mag. 31, 697 (1975).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Charrin, L., Combe, A., Cabane, F. et al. Evidence for the formation of substoichiometric species during internal oxidation of Ag-Mg alloys. Oxid Met 40, 483–501 (1993). https://doi.org/10.1007/BF00666388
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00666388