Abstract
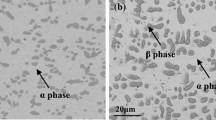
The oxidation behavior of Ag-20Cu-30Cr alloy prepared by powder metallurgy method was investigated in 0.1 MPa pure O2 at 700 and 800 °C. The alloy oxidation kinetics deviates from the parabolic rate law and consists of several quasi-parabolic stages with different rate constants. The alloy oxidation rate at 800 °C is bigger in the initial 4 h and afterward smaller than that at 700 °C up to 24 h. Ag-20Cu-30Cr alloy produces complex oxide scales. At 700 °C, the outer region consists of CuO layer partly covered by metal Ag, while the inner mixed oxidation region consists of alloys and oxides. With the increase of time, an irregular continuous and protective thin layer of Cr2O3 is produced at the base of oxide scales. At 800 °C, the outer region consists of CuO layer, the middle region consists of a metal Ag layer plus a mixed layer of Cu, Ag and Cr oxides or their double oxides, while the inner region consists of a regular continuous and protective thin layer of Cr2O3, which avoids the further oxidation of the alloy.






Similar content being viewed by others
References
C. Wagner, Theoretical Analysis of the Diffusion Process Determining the Oxidation Rate of Alloys, J. Electrochem. Soc., 1952, 99(10), p 369–380.
J.S. Sheasby and D.B. Jory, Electrical Properties of Growing Alumina Scales, Oxid. Met., 1978, 12(5–6), p 527–539.
F. Gesmundo, Y. Niu, F. Viani and D.L. Douglass, The Transition from the Formation of Mixed Scales to the Selective Oxidation of the Most-Reactive Component in the Corrosion of Single and Two-Phase Binary Alloys, Oxid. Met., 1993, 40(3–4), p 373–393.
J. Małecka, Oxidation Behavior of Al2O3 Coating on Ti-25Al-12.5Nb Alloy, J. Mater. Eng. Perform., 2016, 25, p 2951–2958.
N. Birk and H. Richert, The Oxidation Mechanism of Some Nickel-Chromium Alloys, J. JPN. I. Met., 1963, 91(8), p 308–313.
Y. Niu, F. Gesmundo, F. Viani and D.L. Douglass, The Air Oxidation of Two-Phase Cu-Cr Alloys at 700–900 °C, Oxid. Met., 1997, 48(5–6), p 357–380.
G.Y. Fu, Y. Niu and F. Gesmundo, Microstructural Effects on the High Temperature Oxidation of Two-Phase Cu-Cr Alloys in 1 atm O2, Corros. Sci., 2003, 45(3), p 559–574.
F. Gao, S. Wang, F. Gesmundo and Y. Niu, Transitions between Different Oxidation Modes of Binary Cu-Zn Alloys in 0.1 MPa O2 at 1073 K, Oxid. Met., 2008, 69(5–6), p 287–297.
L.Y. Lu, S. Wang, F. Gesmound and Y. Niu, Anomalous Behavior of the Internal Oxidation of Dilute Cu-Ni Alloys under 1 atm O2 at 900 °C, Oxid. Met., 2011, 75(5–6), p 297–311.
F. Gesmundo, F. Viani and Y. Niu, The Possible Scaling Modes in the High-Temperature Oxidation of Two-Phase Binary Alloys. Part II: High Oxidant Pressures, Oxid. Met., 1994, 42(5–6), p 409–429.
F. Gesmundo and Y. Niu, The Internal Oxidation of Ternary Alloys. IV: The Internal Oxidation of the Most Reactive Component beneath External Scales of the Component Having Intermediate Reactivity, Oxid. Met., 2004, 62(5–6), p 357–374.
A. Khan, Y. Huang, Z. Dong and X. Peng, Effect of Cr2O3 nanoparticle Dispersions on Oxidation Kinetics and Phase Transformation of Thermally Grown Alumina on a Nickel Aluminide Coating, Corros. Sci., 2019, 150, p 91–99.
J. Shen, X.H. Guo and Y. Niu, Thermodynamic Phase Diagrams of Ternary Alloys Exposed to a Single Oxidant, Oxid. Met., 2019, 92(3–4), p 195–225.
W.Z. Li, K. Han, R.M. Niu, T.Q. Liang, C.W. Lai and X.H. Zhang, Effect of Si and Y2O3 Additions on the Oxidation Behavior of Ni-xAl (x = 5 or 10 wt%) Alloys at 1150 °C, Oxid. Met., 2018, 89(5–6), p 731–753.
E. Airiskallio, E. Nurmi, M.H. Heinonen, I.J. Väyrynen, K. Kokko, M. Ropo, M.P.J. Punkkinen, H. Pitkänen, M. Alatalo, J. Kollár, B. Johansson and L. Vitos, High Temperature Oxidation of Fe-Al and Fe-Cr-Al Alloys: The Role of Cr as a Chemically Active Element, Corros. Sci., 2010, 52(10), p 3394–3404.
Y. Niu and F. Gesmundo, The Internal Oxidation of Ternary Alloys. VIII: The Transition from the Internal to the External Oxidation of the Two Most-Reactive Components under High-Oxidant Pressures, Oxid. Met., 2006, 65(5–6), p 329–355.
Y. Wu and Y. Niu, High Temperature Scaling of Ni-xSi-10 at.% Al Alloys in 1 atm of Pure O2, Corros. Sci., 2007, 49(3), p 1656–1672.
Y. Niu, S. Wang, F. Gao, Z.G. Zhang and F. Gesmundo, The Nature of The Third-Element Effect in the Oxidation of Fe-xCr-3 at.% Al Alloys in 1 atm O2 at 1000 °C, Corros. Sci., 2008, 50(2), p 345–356.
Z.Q. Cao, Y. Shen, W.H. Liu and R. Xue, Oxidation of Two Three-Phase Cu-30Ni-Cr alloys at 700–800 °C in 1 atm of Pure Oxygen, Mater. Sci. Eng. A, 2006, 425(1–2), p 138–144.
Q.Q. Guo, S. Liu, X.F. Wu, L.L. Liu and Y. Niu, Scaling Behavior of Two Fe-xCr-5Si Alloys under High and Low Oxygen Pressures at 700 °C, Corros. Sci., 2015, 100, p 579–588.
S.Y. Wang, F. Gesmundo and W.T. Wu, A Non-Classical Type of Third-Element Effect in the Oxidation of Cu-xCr-2Al Alloys at 1173 K, Scripta Mater., 2006, 54(9), p 1563–1568.
Z.Q. Cao, C.W. Li, Z.Q. Jia and Y. Wang, High Temperature Corrosion of Nanocrystalline Three-Phase Cu-20Ag-20Cr Bulk Alloy, Corros. Sci., 2016, 110, p 167–172.
J.X. Song, W.T. Wu, Y. Niu and C.L. Wang, Oxidation of Powder Metallurgical Ag-Cr Alloys in 1atm O2 at 700–800 °C, High Temp. Mater. Proc., 2000, 19(2), p 117–125.
C. Wagner, Types of Reaction in the Oxidation of Alloys, Z. Electrochem., 1959, 63, p 772–782.
S. Guruswamy, M. Park, J.P. Hirth and R.A. Rapp, Internal Oxidation of Ag-In Alloys: Stress Relief and the Influence of Imposed Strain, Oxid. Met., 1986, 26(1–2), p 77–100.
Acknowledgments
The authors are grateful for the financial supports from the National Natural Science Foundation of China (51271127 and 22075186), the Liaoning Provincial Key Research and Development Program of China (2018304025), and the Scientific Research Program of Education Department of Liaoning Province of China (LJC201911).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Han, Y., Yu, J., Fan, X. et al. Oxidation Behavior of Ag-20Cu-30Cr Alloy Prepared by Powder Metallurgy Method in 0.1 MPa Pure O2 at 700 and 800 °C. J. of Materi Eng and Perform 30, 9209–9214 (2021). https://doi.org/10.1007/s11665-021-06119-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-021-06119-y