Abstract
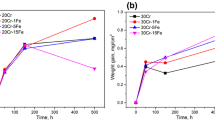
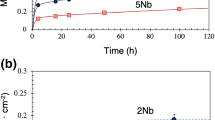
The isothermal oxidation behavior of Ni3Al-0.1B and of this alloy containing additions of approximately 2% Ti, Zr, or Hf was studied in purified oxygen at atmospheric pressure over the temperature range 1300 to 1500 K and for periods up to 400 ks. Ni3Al was also studied similarly for comparison. The oxidation of Ni3Al and Ni3Al-0.1B resulted in an extensive formation of geometric voids on the substrate surface leading to poorly adherent scales. The addition of B to Ni3Al did not change the oxidation behavior much, however, both the activation energy of oxidation and the relative thickness of the Al2O3 layer in the scale were increased. The addition of Ti led to the formation of adherent scales at 1300 K, but the oxidation was accelerated after an initial period at 1300 and 1400 K. At higher temperatures the scale was protective, and the parabolic rate constant, kp, decreased, however, the scale adherence was impaired by the formation of interfacial voids. The addition of Zr resulted in very adherent scales at all temperatures, but at the same time it increased kp considerably. The addition of Hf also resulted in very adherent scales at all temperatures and decreased kp except at 1300 K. The improved scale adherence can be accounted for by the keying effect, since the additives resulted in roughened scale/alloy interfaces. A complex of oxide particle and an associated void was found on the exposed surface of the Hf-containing alloy. This supports the vacancy-sink mechanism.
Similar content being viewed by others
References
S. Taniguchi and T. Shibata,Oxid. Met. in press.
J. D. Kuenzly and D. L. Douglass,Oxid. Met. 8, 139 (1974).
A. Kumar, M. Nasrallah, and D. L. Douglass,Oxid. Met. 4, 227 (1974).
K. Aoki and O. Izumi,J. Japan Inst. Met. 43, 1190 (1979).
C. S. Giggins, B. H. Kear, F. S. Pettit, and J. K. Tien,Met. Trans. 5, 1685 (1974).
J. K. Tien and F. S. Pettit,Met. Trans. 3, 1587 (1972).
R. F. Decker and C. T. Sims, inThe Superalloys, C. T. Sims and W. C. Hagel eds. (John Wiley & Sons, New York, 1972), p. 33.
A. Baldan and D. R. F. West,J. Mat. Sci. 16, 24 (1981).
C. A. Barrett, A. S. Khan, and C. E. Lowell,J. Electrochem. Soc. 128, 25 (1981).
J. L. Pandy, S. Prakash, and M. L. Mehta,Proc. ICMC, 389 (1984).
K. P. R. Reddy, J. L. Smialek, and A. R. Cooper,Oxid. Met. 17, 429 (1982).
I. M. Allam, D. P. Whittle, and J. Stringer,Oxid. Met. 12, 35 (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Taniguchi, S., Shibata, T. & Tsuruoka, H. Isothermal oxidation behavior of Ni3Al-0.1B base alloys containing Ti, Zr, or Hf additions. Oxid Met 26, 1–17 (1986). https://doi.org/10.1007/BF00664270
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00664270