Abstract
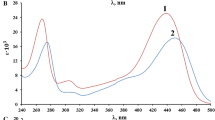
Ultraviolet absorbance spectra of ferric ions in 0.68m NaClO4 were studied as a function of pH at 4.0, 14.9, and 25.0°C. The results provided an evaluation of the stability constant for the formation of FeOH2+ which is *β1=[FeOH +][H +]/[Fe 3+]. The enthalpy change for the reaction Fe3++H2O⇌ FeOH2++H+ was calculated as 10.0±0.3 kcal-mole−1. Increasing temperature was also found to promote the reaction Fe3++2H2O⇌ Fe(OH) +2 +2H+. Our results were combined with the results of other to produce an expression describing the first hydrolysis equilibrium at ionic strengths between 0 and 3m and temperatures between 4.0 and 45.0°C at 1 atm total pressure. At 25°C and 0.68m the ionic strength *β1=1.90×10-3
Similar content being viewed by others
References
D. R. Kester and R. H. Byrne, inFerromanganese Deposits on the Ocean Floor, D. R. Horn, ed., (Columbia University Press, New York, 1972), p. 107.
D. R. Kester, R. H. Byrne, and Y-J. Liang, inMarine Chemistry in the Coastal Environment, T. M. Church, ed., ACS Symposium Series, No. 18 (1975), p. 56.
L. G. Sillen and A. E. Martell,Stability Constants of Metal-Ion Complexes, Special Publication No. 17, The Chemical Society (Burlington House, 1964), p. 53.
L. G. Sillen and A. E. Martell,Stability Constants of Metal-Ion Complexes, Special Publication No. 25, The Chemical Society (Burlington House, 1971), p. 22.
R. H. Byrne and D. R. Kester,Mar. Chem. 4, 255 (1976).
R. H. Byrne and D. R. Kester,Mar. Chem. 4, 275 (1976).
A. B. Lamb and A. G. Jacques,J. Am. Chem. Soc. 60, 1215 (1938).
T. V. Arden,J. Chem. Soc., 350 (1951).
T. Ito and N. Yui, F. Ishikawa Anniversary Volume, Sci. Rep. Tohoku Univ.,37, 19 (1953).
B. O. A. Hedström,Ark. Kemi 6, 1 (1953).
D. D. Perrin,J. Chem. Soc., 1710 (1959).
R. J. Knight and R. N. Sylva,J. Inorg. Nucl. Chem. 37, 779 (1975).
R. S. Sapieszko, R. C. Patel, and E. Matijevié,J. Phys. Chem. 81, 1061 (1977).
R. M. Milburn and W. C. Vosburgh,J. Am. Chem. Soc. 77, 1352 (1955).
R. M. Milburn,J. Am. Chem. Soc. 79, 537 (1957).
A. Barr, J. Goodnight, J. Sall, and J. Helwig,A User's Guide to SAS 76 (Sparks Press, 1976), p. 193.
D. W. Marquardt,J. Soc. Ind. Appl. Math,11, 431 (1963).
T. H. Siddall, III, and W. C. Vosburgh,J. Am. Chem. Soc. 73, 4270 (1951).
W. A. E. McBryde,Analyst 94, 337 (1969).
W. A. E. McBryde,Analyst 96, 739 (1971).
D. H. Richards and K. W. Sykes,J. Chem. Soc., 3626 (1960).
E. Rabinowitch and W. H. Stockmayer,J. Am. Chem. Soc. 64, 335 (1942).
R. Arnek and K. Schlyter,Acta Chem. Scand. 22, 1327 (1968).
O. E. Zvyagintsev and S. B. Lyakhmanov,Russ. J. Inorg. Chem. 13, 643 (1968).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Byrne, R.H., Kester, D.R. Ultraviolet spectroscopic study of ferric hydroxide complexation. J Solution Chem 7, 373–383 (1978). https://doi.org/10.1007/BF00662897
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00662897