Abstract
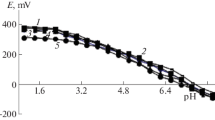
The Clark–Nikolsky oxidation potential is used to study the formation of iron(II) and iron(III) coordination compounds in aqueous solutions of α-alanine at a temperature of 298.15 K and an ionic strength of the (Na(H)ClO4) solution of 1.0 mol/L. Experimental curves of dependences of the EMF of the system on the concentration parameters of hydrogen, iron(III), iron(II), and α-alanine ions (pH, рСох, рСred, and pCL, respectively) are recorded. The curves show that complexation in the studied system proceeds stepwise in the wide range of pH 0.5–9.0. Mononuclear coordination compounds [FeHL(H2O)5]3+, [Fe(HL)2(H2O)4]3+, [Fe2(HL)2(OH)4(H2O)6]2+, [FeHL(H2O)5]2+, [Fe(HL)(OH)(H2O)4]+, and [Fe(HL)(OH)2(H2O)3]0 and a heterovalent [FeIIFeIII(HL)2(OH)4(H2O)6]+ complex form. The type and number of coordinated ligands and cations and the total composition of the resulting complex compounds are determined. Chemical models complexation are compiled, and the regions of their dominance are determined.




Similar content being viewed by others
REFERENCES
L. R. Nozdryukhina, The Biological Role of Trace Elements in the Body of Animals and Humans (Nauka, Moscow, 1977) [in Russian].
T. N. Litvinova, Biogenic Elements. Complex Compounds (Feniks, Rostov-on-Don, 2009) [in Russian].
W. M. Clark, Oxidation-Reduction Potentials of Organic Systems (Williams and Wilkins, Baltimore, 1960).
B. P. Nikol’skii, Oxredmetry (Khimiya, Leningrad, 1975) [in Russian].
M. S. Zakhar’evskii, Oxredmetry (Khimiya, Leningrad, 1968) [in Russian].
M. M. Rakhimova, T. M. Nurmatov, N. Z. Yusupov, M. A. Ismailova, and E. Faizullaev, Russ. J. Inorg. Chem. 58, 719 (2013).
J. A. Davlatshoeva, G. B. Eshova, M. Rahimova, et al., Am. J. Chem. 7, 58 (2017).
G. B. Eshova, J. A. Davlatshoeva, M. Rahimova, L. V. Kvyatkovskaya, and M. O. Guriev, Russ. J. Inorg. Chem. 63, 561 (2018).
G. B. Eshova, J. A. Davlatshoeva, M. Rahimova, M. O. Guriev, and L. V. Kvyatkovskaya, Russ. J. Inorg. Chem. 63, 772 (2018).
M. Rakhimova, G. B. Eshova, D. A. Davlatshoeva, L. V. Kvyatkovskaya, and F. Miraminzoda, Russ. J. Phys. Chem. A 94, 1560 (2020).
J. C. Schumacher, Perchlorates Their Properties, Manufacture and Uses (Nabu Press, USA, 2011).
R. Přibil, Komplexony v chemicke analyze (NCSAV, Praha 1957).
V. L. Zavorotnyi and N. A. Kalacheva, Methodical Guide to Laboratory Work in Analytical Chemistry. Titrimetric Analysis (RGU Nefti Gaza Gubkina, Moscow, 2007) [in Russian].
V. M. Suslennikova and E. K. Kiseleva, Guidelines for the Preparation of Titrated Solutions (Khimiya, Leningrad, 1968) [in Russian].
R. J. Bates, Determination of pH—Theory and Practice (Wiley, New York, 1964).
Z. N. Yusupov, Resp. Tadzhik. Patent TJ 295, No. 97000501, Byull. Izobret., No. 21 (2000).
Funding
This work was performed as part of a project of the Research Institute of the Tajik National University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by M. Timoshinina
Rights and permissions
About this article
Cite this article
Eshova, G.B., Davlatshoeva, D.A., Rakhimova, M. et al. Thermodynamic Characteristics of the Formation of Iron(II) and Iron(III) Complexes with L-Alanine in Aqueous Solutions. Russ. J. Phys. Chem. 97, 2443–2448 (2023). https://doi.org/10.1134/S0036024423110079
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024423110079