Abstract
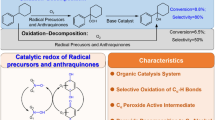
Selective formylation of phenol at the 4-position is achieved by using β-cyclodextrin as catalyst in the reaction of phenol with chloroform in aqueous alkali. The reactions of 1,3-dihydroxybenzene and indol, respectively, in the place of phenol give 2,4-dihydroxybenz-aldehyde and indole-3-aldehyde in virtually 100% selectivies and high yields. The reactions of para-substituted phenols, 4-methylphenol and 5,6,7,8-tetrahydro-2-naphthol, instead of phenol, effect the selective dichloromethylation at the para-positions. Selective carboxylation of phenol at the 4-position is achieved in the reaction of phenol with carbon tetrachloride in aqueous alkali by using β-cyclodextrin and copper powder as catalyst.
The reaction of 2,4,6-trimethylphenol and allyl bromide in aqueous alkali using hexa-N-methylformamido-α-cyclodextrin as catalyst yields 4-allyl-2,4,6-trimethyl-2,5-cyclohexadienone in high selectivity.
The structure of the ternary inclusion complex composed of β-cyclodextrin, phenol, and, chloroform or carbon tetrachloride, formed in the reaction mixture, is determined by NMR spectroscopy. The selective catalysis by cyclodextrin was attributed to the regulation of molecular conformation of substrates with respect to dichlorocarbene, to trichloromethyl cation, or to allyl cation in the ternary molecular complex.
Similar content being viewed by others
References
M. L. Bender and M. Komiyama, ‘Cyclodextrin Chemistry’, Springer-Verlag, West Berlin, 1978.
I. Tabushi, K. Yamamura, K. Fujita, and H. Kawakubo,J. Am. Chem. Soc., 101, 1019 (1979)
M. Ohara and J. Fukuda,Pharmazie, 33, 467 (1978)
M. Komiyama and H. Hirai,Makromol. Chem., Rapid Commun., 2, 715 (1981)
M. Komiyama and H. Hirai,J. Am. Chem. Soc., 105, 2018 (1983)
M. Komiyama and H. Hirai,Makromol. Chem., Rapid Commun., 2, 177 (1981)
M. Komiyama and H. Hirai,J. Am. Chem. Soc., 106, 174 (1984)
M. Komiyama and H. Hirai,Makromol. Chem., Rapid Commun., 2, 661 (1981)
M. Komiyama and H. Hirai,Makromol. Chem., Rapid Commun., 2, 707 (1981)
M. Komiyama and H. Hirai,Makromol. Chem., Rapid Commun., 2, 601 (1981)
M. Komiyama and H. Hirai,Makromol. Chem., Rapid Commun., 2, 757 (1981)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hirai, H. Site-selective C-C bond formation using cyclodextrin as catalyst. Journal of Inclusion Phenomena 2, 455–466 (1984). https://doi.org/10.1007/BF00662212
Issue Date:
DOI: https://doi.org/10.1007/BF00662212