Abstract
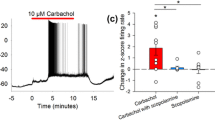
Intracellular recording from neostriatal neurons in rat brain slices revealed effects of the acetylcholine (ACh) agonist carbachol (Cch, 1–10 μmol/l), of the anticholinesterase physiostigmine (10 μmol/l) and of the muscarinic antagonist atropine (10 μmol/l) on plateau potentials elicited in the presence of K-blockers were Cadependent, elicited in the presence of K-blockers were Cadependent, since they persisted in Na-free solution, were resistant to tetrodotoxin (TTX, 3 μmol/l) and blocked by Cd (0.1–0.5 mmol/l). Cch reduced the duration of the plateau potentials and made them more susceptible to fatigue. These effects were antagonized by atropine (1–10 μmol/l), but not by Ba (100–200 μmol/l) or 4-aminopyridine (4-AP, 0.5 mmol/l). Physostigmine (10 μmol/l) had the same atropine-sensitive effects as Cch on the plateau potential. Atropine (10 μmol/l), by itself, prolonged the duration of the plateau potential. High concentrations (100 μmol/l) of Cch did not further reduce the duration of the plateau potential, instead, the duration re-increased with prolonged exposure. The re-increase of the plateau-spike duration was later masked by bursting activity. The opposing effects of low and high concentrations of Cch on the plateau potential duration corresponded to effects of this drug on intrastriatally evoked EPSPs in that low concentrations of Cch reduced the EPSP amplitude, but high concentrations re-increased it after a transient decrease. It is concluded that the muscarinic effect of Ach in the neostriatum is to modulate Ca-influx and that this effect is exerted in a tonic manner.
Similar content being viewed by others
References
Akasu T, Koketsu K (1982) Modulation of voltage-dependent currents by muscarinic receptor in sympathetic neurones of bullfrog. Neurosci Lett 29:41–45
Belluzi O, Sacchi O, Wanke E (1985) Identification of delayed potassium and calcium currents in the rat sympathetic neurone under voltage clmap. J Physiol (Lond) 358:109–129
Bernardi G, Floris V, Marciani MG, Morocutti C, Stanzione P (1976) The action of acetylcholine andl-glutamic acid on rat caudate neurons. Brain Res 114:134–138
Bloom FE, Costa E, Salmoiraghi GC (1965) Anesthesia and the responsiveness of individual neurons of the caudate nucleus of the cat to acetylcholine, norepinephrine and dopamine administered by microelectrophoresis. J Pharmacol Exp Ther 150:244–252
Brown DA (1983) Slow cholinergic excitation—a mechanism for increasing neuronal excitability. TINS 8:302–307
Brown DA, Constanti A (1980) Intracellular observations on the effects of muscarinic agonists on rat sympathetic neurones. Br J Pharmacol 70: 593–608
Brown DA, Selyanko AA (1985) Two components of muscarinesensitive membrane current in rat sympathetic neurones. J Physiol (Lond) 358:335–363
Calabresi P, Misgeld U (1985) Muscarinic modulation of Camediated plateau-spikes in rat neostriatal slices. Neurosci Lett 22:280
Calabresi P, Misgeld U, Dodt HU (1986) Intrinsic membrane properties of neostriatal neurons can account for their low level of spontaneous activity. Neuroscience (in press)
Dodt HU, Misgeld U (1986) Muscarinic slow excitation and muscarinic inhibition of synaptic transmission in the rat neostriatum. J Physiol (Lond) (in press)
Dunlap K, Fischbach GD (1978) Neurotransmitters decrease the calcium component of sensory neurone action potentials. Nature 276:837–839
Dunlap K, Fischbach GD (1981) Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol (Lond) 317:519–535
Egan TM, North RA (1986) Acetylcholine hyperpolarizes central neurones by acting on an M2 muscarinic receptor. Nature 319:405–407
Gähwiler BH, Brown DA (1985) GABAB-receptor-activated K current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Acad Sci 82:1558–1562
Galvan M, Adams PR (1982) Control of calcium current in rat sympathetic neurons by norepinephrine. Brain Res 244:135–144
Hádhazy P, Szerb JC (1977) The effect of cholinergic drugs on3H acetylcholine release from slices of rat hippocampus, striatum and cortex. Brain Res 123:311–322
Hartzell HC, Kuffler SW, Stickgold R, Yoshikami D (1977) Synaptic excitation and inhibition resulting from direct action of acetylcholine on two types of chemoreceptors on individual amphibian parasympathetic neurones. J Physiol (Lond) 271:817–846
Herz A, Zieglgänsberger W (1968) The influence of microelectrophoretically applied biogenic amines, cholinomimetics and procaine on synaptic excitation in the corpus striatum. Int J Neuropharmacol 7:221–230
Horn IP, McAffee DA (1983) Alpha adrenergic inhibition of calcium-dependent potentials in rat sympathetic neurones. J Physiol (Lond) 301:191–204
Horn JP, Dodd J (1981) Monosynaptic muscarinic activation of K+ conductance underlies the slow inhibitory postsynaptic potential in sympathetic ganglia. Nature 292:625–627
Koller WC, Berry CA (1976) Modification of evoked responses in the caudate nucleus by cholinergic agents. Neuropharmacology 15:233–238
Kuba K, Koketsu K (1976) The muscarinic effects of acetylcholine on the action potential of the bullfrog sympathetic ganglion cells. Jpn J Physiol 26:703–716
McCormick DA, Prince DA (1986) Acetylcholine induces burst firing in thalamic reticular neurones by activating a potassium conductance. Nature 319:402–405
McGeer PL, McGeer EG, Fibiger HC, Wickson V (1971) Neostriatal choline acetylase and cholinesterase following selective brain lesions. Brain Res 35:308–314
Misgeld U, Weiler MH, Bak IJ (1980) Intrinsic cholinergic excitation in the rat neostriatum: Nicotinic and muscarinic receptors. Exp Brain Res 39:401–409
Misgeld U, Wagner A, Ohno T (1982a) Depolarizing IPSPs and depolarization by GABA of rat neostriatum cells in vitro. Exp Brain Res 45:108–114
Misgeld U, Weiler MH, Cheong DK (1982b) Astropine enhances nicotinic cholinergic EPSPs in rat neostriatal slices. Brain Res 253:317–320
Misgeld U, Dodt HU, Frotscher M (1986) Late development of intrinsic excitation in the rat neostriatum: An in vitro study. Dev Brain Res 27:59–67
Segal M (1982) Multiple actions of acetylcholine at a muscarinic receptor studied in the rat hippocampal slice. Brain Res 246:77–87
Segal M, Barker JL (1984) Rat hippocampal neurons in culture: Potassium conductances. J Neurophysiol 51:1409–1433
Valentino RJ, Dingledine R (1981) Presynaptic inhibitory effect of acetylcholine in the hippocampus. J Neurosci 1:784–792
Weiler MH, Misgeld U, Bak IJ, Jenden DI (1979) Acetylcholine synthesis in rat neostriatal slices. Brain Res 176:401–406
Weiler MH, Misgeld U, Cheong DK (1984) Presynaptic muscarinic modulation of nicotinic excitation in the rat neostriatum. Brain Res 296:111–120
Zbicz KL, Weight FF (1985) Transient voltage and calcium-dependent outward currents in hippocampal CA3 pyramidal neurons. J Neurophysiol 53:1038–1058
Author information
Authors and Affiliations
Additional information
On leave from absence from: Clinica Neurologica II, Universita di Roma. Tor Vergata, Roma, Italy.
Rights and permissions
About this article
Cite this article
Misgeld, U., Calabresi, P. & Dodt, H.U. Muscarinic modulation of calcium dependent plateau potentials in rat neostriatal neurons. Pflugers Arch. 407, 482–487 (1986). https://doi.org/10.1007/BF00657504
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00657504