Abstract
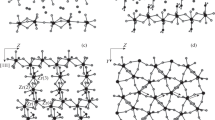
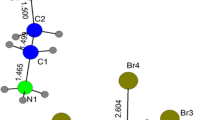
Electron spin resonance (ESR) spectroscopy was used to study the photodecomposition of long-chain diacyl peroxides trapped in channels within zeolites (silicalite and ferrierite) and urea clathrates. ESR spectra of radical pairs in single crystals of the urea clathrates of diundecanoyl peroxide (UP), lauroyl peroxide (LP) andbis(6-bromohexanoyl) peroxide (6-BrHP) show that the alkyl radicals respond to the CO2 stress field by recoiling along the channel. In each clathrate, the inter-radical distance for the most relaxed pair is approx. 9.5 Å, suggesting nearly complete relaxation of stress from the CO2s. The rotational mobility and exceptional kinetic stability of the radicals is attributed to relaxation of stress and the lack of a convenient escape route for the CO2s. X-ray diffraction indicates one-dimensional ordering of guests in 6-BrHP/urea and 3-dimensional ordering of guests in UP/urea. Solid state NMR experiments on LP/urea suggest high guest mobility under ambient conditions. When UP and 6-BrHP were intercalated into silicalite, photolysis yielded isolated radicals, but no radical pairs, even as low as 20 K.
Similar content being viewed by others
References
McBride, J. M.,Accts. Chem. Res.,16, 304 (1983) and refs. cited therein
Bell, J. D. and R. E. Richards,Trans. Farad. Soc.,65, 2529 (1969)
Casal, H. L., D. G. Cameron and E. C. Kelusky,J. Chem. Phys. 80, 1407 (1984)
Griffith, O. H.:J. Chem. Phys. 41, 1093 (1964)
Chatani, Y., H. Anraku and Y. Taki,Mol. Cryst. Liq. Cryst.,48, 219 (1978)
Smith, A. E.:Acta Cryst.,5, 224 (1952)
With UP/urea and 6-BrHP/urea radical pair decay occurs with step-wise kinetics (see reference 8). The half-lives reported here represent the slower rates, which occur at low radical pair concentrations.
Whitsel, B. L., Ph. D. Thesis, Yale University, New Haven, CT. (1977)
see also Mills, D. E., Ph. D. Thesis, Yale University, New Haven, CT. (1986)
Segmuller, B. E., Ph. D. Thesis, Yale University New Haven, CT. (1982)
Pairs of 1,1,2-triphenylethyl radicals formed in single crystals of bis(3,3,3-triphenylpropionyl) peroxide decay with a half-life of 1 min at 256K, but here, intramolecular rearrangement keeps the radical centers isolated from each other. Walter, D. W. and J. M. McBride,J. Am. Chem. Soc.,103, 7069, 7074 (1981)
Hollingsworth, M. D., Ph. D. Thesis, Yale University, New Haven, CT. (1986)
McBride, J. M., M. W. Vary and B. L. Whitsel,ACS Symp. Ser.,69, 208 (1978)
See Hollingsworth, M. D. and J. M. McBride,J. Am. Chem. Soc.,107, 1792 (1985) for an related case in which a distant defect allows relaxation of stress.
Casal, H. L. and J. C. Scaiano,Can. J. Chem.,62, 628 (1984)
Meier, W. M. and D. H. Olson,Atlas of Zeolite Structure Types, Structure Commission of the International Zeolite Association, Zurich (1978)
At the time of submission, the first two control experiments had not been done for 6-BrHP/ferrierite.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hollingsworth, M.D., Harris, K.D.M., Jones, W. et al. ESR and X-ray diffraction studies of diacyl peroxides in urea and aluminosilicate hosts. Journal of Inclusion Phenomena 5, 273–277 (1987). https://doi.org/10.1007/BF00655664
Issue Date:
DOI: https://doi.org/10.1007/BF00655664