Abstract
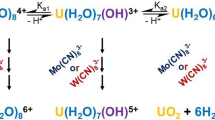
The solubility of carefully characterized UO2 in pOH 1.5 and pOH 2.5 aqueous solutions has been determined from 25°C to 300°C using a flow apparatus. Data were analyzed in terms of the reaction
The extreme sensitivity of both the UO2 surface and aqueous U(IV) to oxidation is discussed.
Similar content being viewed by others
References
D. Langmuir,Geochim. Cosmochim. Acta 42, 547 (1978).
R. J. Lemire and P. R. Tremaine,J. Chem. Eng. Data 25, 361 (1980).
C. F. Baes, Jr. and R. E. Mesmer,The Hydrolysis of Cations, (Wiley Interscience, New York, 1976).
N. M. Nikolaeva,Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Khim. Nauk 4, 91 (1978).
K. H. Gayer and H. Leider,Can. J. Chem. 35, 5 (1957).
L. Lynds, W. A. Young, J. S. Mohl, and G. G. Libowitz, inNonstoichiometric Compounds, Advan. Chem. Ser. 39, R. F. Gould, ed. (American Chemical Society, 1962), Chapter 5.
N. S. McIntyre, S. Sunder, D. W. Shoesmith, and F. Stanchell,J. Vac. Sci. Technol., in press.
P. R. Tremaine and J. C. LeBlanc,J. Solution Chem. 9, 415 (1980).
J. P. de Kleijn and B. van Zanten,J. Radioanalyt. Chem. 45, 195 (1978).
G. C. Allen, J. A. Crofts, M. T. Curtis, P. M. Tucker, D. Chadwick, and P. J. Hampson,J. Chem. Soc., Dalton Trans., 1296 (1974).
J. Verbist, J. Riga, J. J. Pireaux, and R. Caudano,J. Electron Spectros. Relat. Phenom. 5, 193 (1974).
J. S. Anderson, L. E. J. Roberts, and E. A. Harper,J. Chem. Soc., 3946 (1955).
L. W. Neidrach,Science 207, 1200 (1980) andGeneral Electric Tech. Inform. Ser. 79CRD265 (1980).
P. Schindler, inEquilibrium Concepts in Natural Water Systems, Advan. Chem. Ser. 67, R. F. Gould, ed. (American Chemical Society, 1967), Chapter 9.
C. Keller, inThe Chemistry of the Actinides by S. Ahrland, et al. (Pergamon Press, New York, 1975), p. 248.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tremaine, P.R., Chen, J.D., Wallace, G.J. et al. Solubility of uranium (IV) oxide in alkaline aqueous solutions to 300°C. J Solution Chem 10, 221–230 (1981). https://doi.org/10.1007/BF00653099
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00653099