Abstract
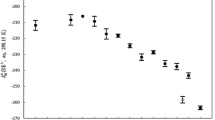
The apparent molal volumes of aqueous ZnCl2 and Zn(ClO4)2 solutions have been measured from 15–55°C. The dilute solution data are extrapolated to infinite dilution using the Redlich-Meyer equation. The full concentration range data are fitted with the Pitzer formalism. The data are then compared with the data on the previously measured salts of Mn2+, Fe2+, Co2+, Ni2+, and Cu2+. The effect of complex ion formation is easily seen in the Cu2+ and Zn2+ salt data. A new approach to single ion volumes from salt volumes is proposed. The calculated ionic volumes at infinite dilution are compared, and it is clear that crystal field effects must be considered in any quantitative theory of transition element volumes.
Similar content being viewed by others
References
R. Pogue and G. Atkinson,J. Chem. Eng. Data 33, 370 (1988).
R. Pogue and G. Atkinson,J. Chem. Eng. Data (in press).
G. Schwarzenbach and H. Flaschka,Complexometric Titrations (Methuen, London, 1969).
F. J. Millero,Water and Aqueous Solutions: Structure, Thermodynamics, and Transport Processes (Wiley Interscience, New York, 1972).
O. Redlich and D. Meyer,Chem. Rev. 64, 221 (1964)
O. Redlich,J. Phys. Chem. 67, 496 (1963).
T. Herrington, M. Roffey, and D. Smith,J. Chem. Eng. Data 31, 221 (1986).
W. Grzybkowski and G. Atkinson,J. Chem. Eng. Data 31, 309 (1986).
B. Conway, R. Verrall, and J. Desnoyers,J. Trans. Faraday Soc. 62, 2738 (1966).
R. Zana and E. Yeager,J. Phys. Chem. 70, 954 (1966)
R. Zana and E. Yeager,J. Phys. Chem. 71, 4241 (1967).
P. Drude and W. Nernst,Z. Phys. Chem. (Frankfurt) 15, 79 (1894).
R. Pogue, Ph.D. Dissertation, University of Oklahoma, 1988.
K. Pitzer and P. Rogers,J. Phys. Chem. Ref. Data 11, 15 (1982).
L. Pauling,The Nature of the Chemical Bond (Cornell University Press, New York, 1940).
A. Couture and K. Laidler,Can. J. Chem. 34, 1209 (1956).
F. Millero,J. Phys. Chem. 75, 280 (1971).
R. Noyes,J. Am. Chem. Soc. 86, 971 (1964).
J. Padova,J. Chem. Phys. 39, 1552 (1963).
R. Smith and A. Martell,Critical Stability Constants 4: Inorganic Complexes (Plenum Press, New York, 1976).
L. Orgel and J. Dunitz,Nature 179, 462 (1957).
J. Spitzer, I. Olofsson, P. Singh, and L. Hepler,J. Chem. Thermodyn. 11, 233 (1979).
F. Millero and A. LoSurdo,J. Phys. Chem. 84, 710 (1980).
J. Spitzer, I. Olofsson, P. Singh, and L. Hepler,J. Solution Chem. 7, 623 (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pogue, R.F., Atkinson, G. Solution thermodynamics of first row transition elements. 3. Apparent molal volumes of aqueous ZnCl2 and Zn(CIO4)2 from 15 to 55°C and an examination of solute-solute and solute-solvent interactions. J Solution Chem 18, 249–264 (1989). https://doi.org/10.1007/BF00652987
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00652987