Abstract
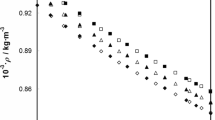
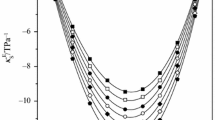
Isobaric thermal expansion αp, and isothermal compressibility κp have been determined for binary mixtures of ethylbenzene + n-hexane, and + n-octane in the complete range of composition at 25 and 45°C. The corresponding excess quantities obtained at each measured mole fraction are negative for both systems and show minima at or around equimolecular composition. Absolute values of those excess functions decrease with the chain length of the n-alkane and increase with temperature. Combining these experimental results with data for the heat capacity at constant pressure the isentropic compressibility and the heat capacity at constant volume were calculated at 25°C. The corresponding excess quantities are negative, showing maxima around mole fraction 0.5 and their absolute values decrease for κ ES and increase for ΔC V with increasing chain length.
Similar content being viewed by others
References
G. Tardajos, E. Aicart, M. Costas, and D. Patterson,J. Chem. Soc. Faraday Trans. l. 82, 2977 (1986).
E. Aicart, G. Tardajos, and M. Díaz Peña,J. Chem. Thermodyn. 13, 783 (1981).
J. Garbajosa, G. Tardajos, E. Aicart, and M. Díaz Peña,J. Chem. Thermodyn. 14, 671 (1982)
I. Gamboa, G. Tardajos, M. Diaz Pena, and E. Aicart,J. Chem. Thermodyn. 18, 885 (1986).
R. L. Arenosa, R. G. Rubio, C. Menduiña, and M. Díaz Peña,J. Solution Chem. 14, 345 (1985).
M. Cáceres, J. M. Arsuaga, and J. Núñez,Fluid Phase Equilibria 20, 81 (1985).
M. Díaz Peña and M. C. McGlashan,Trans. Faraday Soc. 57, 1511 (1961)
M. Díaz Peña and G. Tardajos,J. Chem. Thermodyn. 10, 19 (1978).
F. D. Rossini, et al.,Selected Values of Physical Thermodynamic Properties of Hydrocarbons and Related Compounds, API Research Project 44, (Carnegie Press, Pittsburgh, PA 1953).
A. Abe and P. J. Flory,J. Amer. Chem. Soc. 87, 1838 (1964).
M. B. Ewing and K. N. Marsh,J. Chem. Thermodyn. 9, 371 (1977).
J. L. Fortier and G. C. Benson,J. Chem. Eng. Data 25, 47 (1980).
J. F. Messerly, G. B. Guthrie, S. S. Todd, and H. L. Finke,J. Chem. Eng. Data 12, 338 (1967).
J. P. E. Grolier, A. Faradjzadeh, and H. V. Kehiaian,Thermochimica Acta 53, 157 (1982).
Y. P. Handa, C. J. Halpin, and G. C. Benson,J. Chem. Thermodyn. 13, 875 (1981).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Matilla, A.D., Aicart, E., Peña, M.D. et al. Isobaric thermal expansion and isothermal compressibility of ethylbenzene + n-hexane, and + n-octane at 25 and 45°C. J Solution Chem 18, 143–150 (1989). https://doi.org/10.1007/BF00649570
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00649570