Abstract
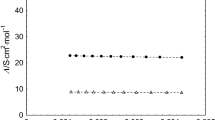
The apparent and partial molar volumes in aqueous solution were obtained for (n-Bu)4PBr and (n-Bu)4-n Ph n PCl (n=1–4) at six temperatures from 1 to 55°C. The apparent molar expansibilities were also obtained. The hydrophobic character of the cations is reduced by replacing butyl groups with phenyl groups, as evidenced by the decrease in the magnitudes of the B v -coefficient (negative for all n) and of the temperature dependent extrema found in the apparent molar volumes and expansibilities as a function of concentration. However, the extrema exist even with BuPh3PCl at low temperatures. The result suggests that the phenyl groups weakly affect the butyl cospheres and cation-cation interactions.
Similar content being viewed by others
References
H. L. Friedman and C. V. Krishnan, inWater: A Comprehensive Treatise, Vol. 3 F. Franks, ed., (Plenum Press, New York and London, 1973), pp. 1
G. Kalfoglou and L. H. Bowen,J. Phys. Chem. 73, 2728 (1969).
C. Jolicoeur, P. T. Philip, G. Perron, P. A. Leduc, and J. E. Desnoyers,Can. J. Chem. 50, 3167 (1972).
A. Bondi,J. Phys. Chem. 68, 441 (1964).
K. Takaizumi and T. Wakabayashi,J. Solution Chem. 11, 809 (1980).
K. Takaizumi and T. Wakabayashi,J. Solution Chem. 11, 819 (1980).
T. Wakabayashi and K. Takaizumi,Bull. Chem. Soc. Japan 54, 951 (1981).
G. S. Kell,J. Chem. Eng. Data 12, 66 (1967).
O. Redlich and D. M. Meyer,Chem. Rev. 64, 221 (1964).
G. Beurskens, G. A. Jeffrey, and R. K. McMullan,J. Chem. Phys. 39, 3311 (1963).
G. Perron, N. Desrosiers, and J. E. Desnoyers,Can. J. Chem. 54, 2163 (1976).
L. Hepler,Can. J. Chem 47, 4613 (1969).
J. L. Neal and D. A. Goring,J. Phys. Chem. 74, 658 (1970).
R. McMullan and G. A. Jeffrey,J. Chem. Phys. 31, 1231 (1959).
W. Y. Wen and S. Saito,J. Phys. Chem. 68, 2639 (1964);ibid.,69, 3569 (1965).
W. Y. Wen and K. Nara,J. Phys. Chem. 71, 3907 (1967).
F. Franks and H. S. Smith,Trans. Faraday Soc. 63, 2586 (1967).
T. L. Broadwater and D. F. Evans,J. Phys. Chem. 73, 164 (1969).
W. Y. Wen, K. Nara, and R. H. Wood,J. Phys. Chem. 72, 3049 (1968).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wakabayashi, T., Takaizumi, K. Apparent and partial molar volumes of quaternary phosphonium halides in aqueous solutions. J Solution Chem 11, 565–579 (1982). https://doi.org/10.1007/BF00649257
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00649257