Abstract
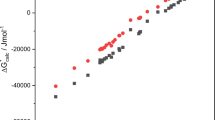
An attempt has been made to parameterize the structuredness of solvents from the viewpoint of intermolecular interactions, and the structuredness parameter S p has newly been proposed. The enthalpy of vaporization ΔH /o vap of various solvents has been considered to consist of donor-acceptor interaction energy (DA), which can been estimated from Gutmann's donor and acceptor numbers, some other interaction energies (VDW), which may not be fully described in terms of the donor-acceptor interactions and may be related to the electronic distribution, the volume and shape of the molecule, the polarizability and ionization potential of atoms in the molecule, the energies of these interactions being usually considered to be of Van der Waals type and possibly evaluated from the enthalpy of vaporization ofn-alkanes, and the intermolecular interaction energy (STR) due to the three-dimensional molecular ordering in the liquid: ΔH /o vap =DA+VDW+STR. The STR term obtained as the difference between ΔH /o vap and (DA+VDW) is defined as the structuredness parameter S p , which is a dimensionless quantity by dividing the value with the (kJ-mol−1) unit. The entropies of formation ΔS o1 and ΔS oβ4 of [MX]+ and [MX4]2− complexes, respectively, of divalent metal ions (Mn2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, and Hg2+) with halide and thiocyanate ions in aqueous and nonaqueous solvents could be represented as an almost linear function of the structuredness parameters S p .
Similar content being viewed by others
References
V. Gutmann and E. Wychera,Inorg. Nucl. Chem. Lett. 2, 257 (1966); V. Gutmann,Coordination Chemistry in Nonaqueous Solutions (Springer-Verlag, New York, 1968).
U. Mayer, V. Gutmann, and W. Gerger,Mh. Chem. 106, 1235 (1975).
K. Dimroth, C. Reichardt, T. Siepmann, and F. Bohlmann,Justus Liebigs Ann. Chem. 661, 1 (1963); C. Reichardt,Angew. Chem. Int. Ed. Eng. 4, 29 (1965).
E. M. Kosower,J. Am. Chem. Soc. 80, 3253, 3261, 3267 (1958).
J. S. Berson, Z. Hamlet, and W. A. Mueller,J. Am. Chem. Soc. 84, 297 (1962).
R. S. Drago,Structure and Bonding 15, 73 (1973).
H. Ohtaki, A. Funaki, B. M. Rode, and G. Reibnegger,Bull. Chem. Soc. Jpn. 56, 2116 (1983); H. Ohtaki and S. Itoh,Z. Naturforsch. 40a, 1351 (1985).
H. Ohtaki, S. Itoh, and B. M. Rode,Bull. Chem. Soc. Jpn. 59, 271 (1986).
S. Itoh and H. Ohtaki,Z. Naturforsch. 42a, 858 (1987).
H. Ohtaki, S. Itoh, T. Yamaguchi, S. Ishiguro, and B. M. Rode,Bull. Chem. Soc. Jpn. 56, 3406 (1983).
A. Kratochwill, J. U. Weidner, and H. Zimmermann,Ber. Bunsen Ges. 77, 408 (1973); H. Bertagnolli and M. Zeidler,Mol. Phys. 36, 177 (1978); T. Radnai, S. Itoh, and H. Ohtaki,Bull. Chem. Soc. Jpn. 61, 3845 (1988).
T. Radnai, S. Ishiguro, and H. Ohtaki,J. Solution Chem. 18, 771 (1989).
V. Gutmann,Electrochim. Acta 21, 661 (1976).
D. Eisenberg and W. Kauzmann,The Structure and Properties of Water (Clarendon Press, London, 1969).
Chemical Society of Japan, ed.,Kagaku Binran (Handbook of Chemistry) 3rd end., (Maruzen, Tokyo, 1984); J. A. Riddle and W. B. Bunger,Organic Solvents, 3rd end., (Wiley, New York, 1970); C. H. Rochester and J. R. Symonds,J. Chem. Soc. Faraday Trans. I 69, 1267 (1973).
The entropy data are taken form the following papers: S. Ishiguro, B. G. Jeliazkova, and H. Ohtaki,Bull. Chem. Soc. Jpn. 58, 1143, 1749 (1985); S. Ishiguro, H. Suzuki, B. G. Jeliazkova, and H. Ohtaki,Bull. Chem. Soc. Jpn. 59, 2407 (1986); S. Ishiguro, K. Ozutsumi, and H. Ohtaki,Bull. Chem. Soc. Jpn. 60, 531 (1987);J. Chem. Soc. Faraday Trans. I 84, 2409 (1988); H. Suzuki, S. Ishiguro, and H. Ohtaki,J. Chem. Soc. Faraday Trans. I 86, 2179 (1990); S. Ahrland, N.-O. Björk, and R. Portanova,Acta Chem. Scand. A30, 270 (1976); S. Ahrland, N.-O. Björk, and I. Persson,Acta Chem. Scand. A35, 67 (1981); P. Gerding,Acta Chem. Scand. 20, 79 (1966);22, 1283 (1968);23, 1695 (1969); A. H. Zeltmann, N. A. Matwiyoff, and L. O. Morgan,J. Phys. Chem. 72, 121 (1968); S. Ahrland and N.-O. Björk,Acta Chem. Scand. A30, 257 (1976); C. E. Vanderzee and H. J. Dawson,J. Am. Chem. Soc. 75, 5659 (1953); P. Gerding and I. Jönsson,Acta Chem. Scand. 22, 2247 (1968); P. Kivalo and P. Ekari,Suomen Kem. B30, 116 (1957); L. Ciavatta and M. Grimaldi,J. Inorg. Nucl. Chem. 30, 197 (1968);Inorg. Chim. Acta 4, 312 (1970); P. K. Gallagher, Thesis, Univerisity of Wisconsin (1960); P. K. Gallagher and E. L. King,J. Am. Chem. Soc. 82, 3510 (1960); R. Arnek,Arkiv Kem. 24, 531 91965); Y. Marcus,Acta Chem. Scand. 11, 599 91957); J. J. Christensen, R. M. Izatt, L. D. Hansen, and J. D. Dale,Inorg. Chem. 3, 130 (1964); S. Ahrland, I. Persson, and R. Portanova,Acta Chem. Scand. A35, 49 (1981); S. Ahrland and L. Kullberg,Acta Chem. Scand. 25, 3692 (1971); S. Ishiguro, K. Yamamoto, and H. Ohtaki,Bull. Chem. Soc. Jpn. 59, 1009 (1986); S. Ishiguro, T. Takamuku, and H. Ohtaki,Bull. Chem. Soc. Jpn. 61, 3901 (1988).
S. Yamada and M. Tanaka,J. Inorg. Nucl. Chem. 37, 587 (1975).
T. Yamaguchi, K. Yamamoto, and H. Ohtaki,Bull. Chem. Soc. Jpn. 58, 3235 (1985).
K. Ozutsumi, T. Takamuku, S. Ishiguro, and H. Ohtaki,Bull. Chem. Soc. Jpn. 62, 1875 (1989).
Unpublished data.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ohtaki, H. An attempt to parameterize the structuredness of solvents. J Solution Chem 21, 39–47 (1992). https://doi.org/10.1007/BF00648979
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00648979