Abstract
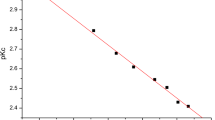
The molar conductances of dilute solutions of salicylic and monochloroacetic acids in binary mixtures of methanol, ethanol, 1-propanol, N,N-dimethylformamide, acetone, acetonitrile, tetrahydrofuran and dioxane with water have been measured at 25°C. The Lee-Wheaton conductance equation was fitted to the data in order to derive thermodynamic dissociation constants and limiting molar conductances. The results were compared with those in the literature pertaining to analogous media, mostly derived potentiometrically. The findings are interpreted in terms of a solvent effect on the ionization of these acids in mixed solvent systems.
Similar content being viewed by others
References
M. S. K. Niazi and J. Mollin,Bull. Chem. Soc. Jpn. 60, 2605 (1987).
M. S. K. Niazi,Bull. Chem. Soc. Jpn. 62, 1253 (1989).
M. S. K. Niazi, S. S. Shah, J. Ali, and M. Z. I. Khan,J. Solution Chem. 19, 623 (1990).
M. S. K. Niazi and J. Ali,Bull. Chem. Soc. Jpn. 63, 3619 (1990).
O. Fischer, M. S. K. Niazi, and E. Fischerova,Electrochim. Acta 27, 791 (1982).
M. S. K. Niazi, O. Fischer, and E. Fischerova,J. Solution Chem. 15, 957 (1986).
M. S. K. Niazi,Bull. Chem. Soc. Jpn. 61, 2165 (1988).
D. N. Bhattachayya and C. L. Lee,J. Phys. Chem. 69, 608 (1965).
A. D'Aprano, F. Accascina, and R. M. Fuoss,J. Solution Chem. 19, 65 (1990).
J. E. Lind, J. J. Zwolenik, and R. M. Fuoss,J. Am. Chem. Soc. 81, 1557 (1959).
G. Akerlof,J. Am. Chem. Soc. 54, 4125 (1932).
C. Moreau and G. Douheret,J. Chem. Thermodyn. 8, 403 (1976);Thermochimica. Acta 13, 385 (1975).
R. Reynaud,C. R. Acad. Sci. Paris 266C, 489 (1968);263C, 905 (1966).
G. Akerlof and O. A. Short,J. Am. Chem. Soc. 58, 1241 (1936).
F. E. Critchfield, J. A. Gibbon, and J. L. Hall,J. Am. Chem. Soc. 75, 6044 (1953).
J. A. Geddes,J. Am. Chem. Soc. 55, 4832 (1933).
W. H. Lee and R. J. Wheaton,J. Chem. Soc. Faraday Trans. II 74, 743 (1978).
A. D. Pethybridge and S. S. Taba,J. Chem. Soc. Faraday Trans. I 76, 368 (1980).
R. M. FuossJ. Phys. Chem. 79, 525 (1975);82, 2427 (1978).
F. Bradley and W. C. M. Lewis,J. Phys. Chem. 29, 782 (1925).
N. Papadopoulis and A. Avrenas,J. Solution Chem. 20, 293 (1991).
L. G. Bray, J. F. J. Dippy, S. R. C. Hughes, and L. W. Laxton,J. Chem. Soc. 2405 (1957).
A. K. Mandal and S. C. Lahiri,Bull. Chem. Soc. Jpn. 49, 1829 (1976).
B. Saxton and T. W. Langer,J. Am. Chem. Soc. 55, 3636 (1933).
S. K. Chakraborty, S. K. Maity and S. C. Lahiri,Z. Phys. Chem. (Liepzig) 266, 1194 (1985).
R. L. Kay and T. L. Broadwater,J. Solution Chem. 5, 57 (1976).
E. Grunwald and B. J. Berkowitz,J. Am. Chem. Soc. 73, 4939 (1951).
J. H. Elliot and M. Kilpatrick,J. Phys. Chem. 45, 485 (1941).
S. R. Singh, A. K. Tandon, and S. P. Rao,Z. Phys. Chem. 255, 109 (1974).
G. Poulias, N. Papadopolous, and D. Jannakoudakis,Electrochim. Acta 30, 431 (1985).
J. F. J. Dippy, S. R. C. Hughes, and B. C. Kitchiner,J. Chem. Soc. 1275 (1964).
J. O. Halford,J. Am. Chem. Soc. 55, 2272 (1933).
O. Lukkari, E. Hakoila, and L. Markkola,Finn. Chem. Lett. 93 (1978).
S. P. Rao, B. Sitaram, and R. V. Reddy,Indian J. Chem. 20A, 485 (1981).
S. M. Petrov, Yu. I. Umanskii, N. D. Golikova, F. S. Khabibova, and L. A. Safiullina,Russ. J. Phys. Chem. (Eng.) 43, 1670 (1969).
R. G. Bates and Z. Pawlak,J. Solution Chem. 5, 213 (1976).
Author information
Authors and Affiliations
Additional information
This paper is dedicated to the late Professor Raymond M. Fuoss on his 3rd anniversary.
Rights and permissions
About this article
Cite this article
Niazi, M.S.K., Khan, M.Z.I. Thermodynamic dissociation constants of salicylic and monochloroacetic acids in mixed solvent systems from conductance measurements at 25°C. J Solution Chem 22, 437–456 (1993). https://doi.org/10.1007/BF00647681
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00647681