Abstract
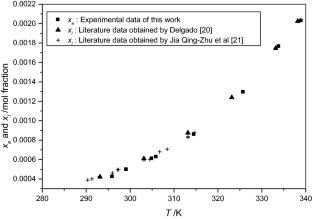
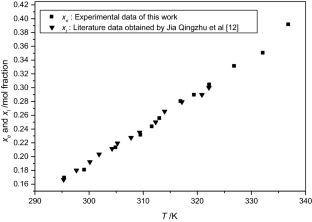
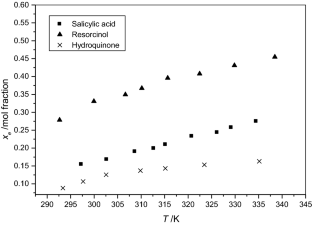
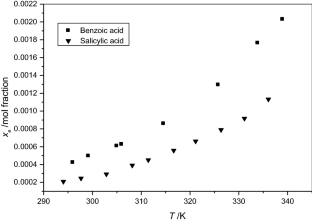
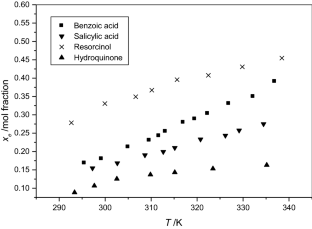
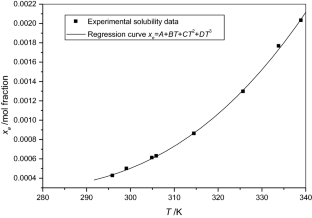
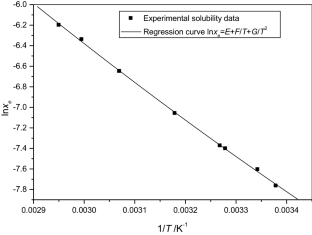
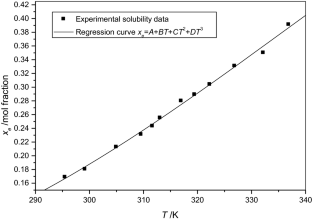
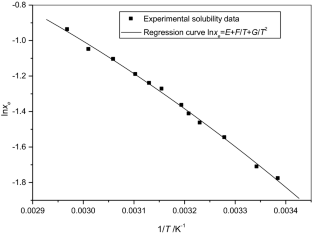
The solubilities of benzoic acid, salicylic acid, resorcinol and hydroquinone in water and in 1-octanol were measured by the dynamic method which is also called the synthetic method from 297.25 K to 334.45 K. Using differential scanning calorimetry (DSC Q2000 and SDT Q600), the melting temperature and the enthalpy of fusion of these solutes were determined. The obtained results show that the solubility of benzoic acid in water is greater than that of salicylic acid, but in the case of the two isomers of dihydroxybenzene, the solubility of resorcinol is approximately 100 times that of hydroquinone. In 1-octanol, the decreasing order of the solubility of these compounds is as follows: resorcinol > benzoic acid > salicylic acid > hydroquinone. The experimental solubilities were correlated using two regression equations. The correlation coefficient is greater than 0.9937 for one of these two equations for the binary studied systems where the solvent is either water or 1-octanol. New experimental data are provided for the solubility of resorcinol in water and salicylic acid, resorcinol, hydroquinone in 1-octanol.

Similar content being viewed by others
References
C. M. S. de Mendonça, I. P. de Barros Lima, C. F. S. Aragão, A. P. B. Gomes, J. Therm. Anal. Calorim. 115, 2277–2285 (2014)
A.-C. Huang, Y.-K. Chuang, C.-F. Huang, C.-M. Shu, J. Therm. Anal. Calorim. 132, 165–172 (2018)
X. Li, Q. Yin, W. Chen, J. Wang, J. Chem. Eng. Data 51, 127–129 (2006)
J. Lim, S. Jang, H. K. Cho, M. S. Shin, H. Kim, J. Chem. Thermodyn. 57, 295–300 (2013)
R. F. Pires, M. R. Franco Jr, Fluid Phase Equilib. 330, 48–51 (2012)
A. Shalmashi, A. Eliassi, J. Chem. Eng. Data 53, 199–200 (2008)
N. Sunsandee, S. Suren, N. Leepipatpiboon, M. Hronec, U. Pancharoen, Fluid Phase Equilib. 338, 217–223 (2013)
S. H. Ali, F. S. Al-Mutairi, M. A. Fahim, Fluid Phase Equilib. 230, 176–183 (2005)
A. Apelblat, E. Manzurola, N. A. Balal, J. Chem. Thermodyn. 38, 565–571 (2006)
K. Carlsson, B. Karlberg, Anal. Chim. Acta 423, 137–144 (2000)
S.-T. Lin, S. I. Sandler, Ind. Eng. Chem. Res. 38, 4081–4091 (1999)
J. Qingzhu, M. Peisheng, Y. Shouzhi, W. Qiang, W. Chang, L. Guiju, J. Chem. Eng. Data 53, 1278–1282 (2008)
A. Van Haelst, P. Heesen, F. Van Der Wielen, H. Govers, Chemosphere 29, 1651–1660 (1994)
W. J. Weber Jr, Y.-P. Chin, C. P. Rice, Water Res. 20, 1433–1442 (1986)
G. Wienke, J. Gmehling, Toxicol. Environ. Chem. 65, 57–86 (1998)
Y.-H. Zhang, Trends Analyt. Chem. 15, 188–196 (1996)
U. Domańska, Fluid Phase Equilib. 114, 175–188 (1996)
I. Hahnenkamp, G. Graubner, J. Gmehling, Int. J. Pharm. 388, 73–81 (2010)
H. Li, G. Hu, F. Guo, L. Zhao, J. Zhu, Y. Zhang, Can. J. Chem. Eng. 88, 161–164 (2010)
J. Delgado, Int. J. Heat Mass Transf. 43, 1311–1316 (2007)
J. Qing-Zhu, M. Pei-Sheng, Z. Huan, X. Shu-Qian, W. Qiang, Q. Yan, Fluid Phase Equilib. 250, 165–172 (2006)
F. L. Mota, A. J. Queimada, A. E. Andreatta, S. P. Pinho, E. A. Macedo, Fluid Phase Equilib. 322, 48–55 (2012)
K. Tamura, T. Kasuga, T. Nakagawa, Fluid Phase Equilib. 420, 24–29 (2016)
W. E. Acree Jr, Thermochim Acta 189, 37–56 (1991)
F. L. Nordström, Å. C. Rasmuson, J. Chem. Eng. Data 51, 1668–1671 (2006)
C. L. Yaws, S.-C. Lin, Enthalpy of fusion at freezing point—Organic compounds, in Thermophysical Properties of Chemicals and Hydrocarbons, William Andrew Publishing (Elsevier, 2009), pp. 552–591
Z. Esina, M. Korchuganova, Theor. Found. Chem. Eng. 49, 313–321 (2015)
Funding
This study was funded by Ministère de l'Enseignement Supérieur et de la Recherche Scientifique.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Belhachemi, B., Makhlouf, H. & Belhachemi, M.H. Determination and Correlation of Solubilities of Benzoic Acid, Salicylic Acid, Resorcinol and Hydroquinone in Water and in 1-Octanol at Temperatures from 297.25 K to 334.45 K. Int J Thermophys 42, 1 (2021). https://doi.org/10.1007/s10765-020-02750-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-020-02750-4