Abstract
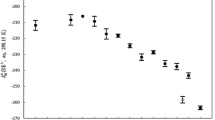
Solvent isotope effects on differential heats of solution of NaCl in HOH and DOD have been measured between 0.01 and 4m and from 5 to 40°C. These data are supplemented by integral heats of solution measured at 0.465m and from 5 to 75°C, and with heats of dilution over the same temperature range. The integral heat measurements include studies on otheralkali halides (KCl, NaI, NaBr), and the dilution measurements include some data for KCl. In the NaCl studies, particular attention was paid to the low-concentration region. Values of the partial molal enthalpiesL 1 adL 2 are derived for solution in both isotopic waters, and new values for the isotope effect on the standard enthalpy of solution ΔL s2 = (ΔH 02 ) d←w as a function of temperature are reported. Comparisons are made with previous work where possible. In contrast to statements made by earlier authors, examination of the low-concentration data reveals a\(\surd \overline m\) dependence within experimental error.
Similar content being viewed by others
References
Water. A Comprehensive Treatise, F. Franks, ed., Vols. 1, 2, 3 (Plenum Press, New York, 1973).
E. M. Arnett and D. R. McKelvey, inSolute-Solvent Interactions, J. F. Coetzee and C. D. Ritchie, eds. (Interscience Publishers, New York, 1969), pp. 343–398.
H. L. Friedman and C. V. Krishnan, inWater. A Comprehensive Treatise, Vol. 3, F. Franks, ed. (Plenum Press, New York, 1973), pp. 1–118.
J. Bigeleisen, M. W. Lee, and F. Mandel,Ann. Rev. Phys. Chem. 24, 407 (1973).
G. Jancso and W. A. Van Hook,Chem. Rev. 74, 689 (1974).
L. I. Kleinman and M. Wolfsberg,J. Chem. Phys.,59, 2043 (1973);60, 4740, 4749 (1974).
A. Y. Fang and W. A. Van Hook,J. Chem. Phys. 60, 3513 (1974).
J. Bigeleisen,J. Chim. Phys. 61, 87 (1964).
J. Pupezin, G. Jakli, G. Jancso, and W. A. Van Hook,J. Phys. Chem. 76, 743 (1972).
G. Jakli, T. C. Chan, and W. A. Van Hook,J. Solution Chem. 4, 71 (1975).
M. Y. Schrier and E. E. Schrier,J. Chem. Thermodyn. 5, 811 (1973).
R. E. Kerwin, Ph.D. Dissertation, University of Pittsburgh, 1964.
O. D. Bonner,J. Chem. Thermodyn. 3, 837 (1971).
P. R. Marshall and J. J. Katz,J. Inorg. Nucl. Chem. 36, 1589 (1974).
R. A. Robinson,J. Phys. Chem. 73, 3165 (1969).
R. A. Robinson,J. Solution Chem. 5, 819 (1973).
Y. C. Wu and H. L. Friedman,J. Phys. Chem. 70, 166 (1966).
P. R. Philip and J. R. Desnoyers,J. Solution Chem. 1, 353 (1972).
J. E. Desnoyers, R. Francescon, P. Picker, and C. Jolicoeur,Can. J. Chem. 49, 3460 (1971).
P. R. Philip, G. Perron, and J. E. Desnoyers,Can. J. Chem. 52, 1709 (1974).
J. L. Fortier, P. R. Philip, and J. E. Desnoyers,J. Solution Chem. 3, 523 (1974).
R. H. Wood, R. A. Rooney, and J. N. Braddock,J. Phys. Chem. 73, 1673 (1969).
A. A. Levine and R. H. Wood,J. Phys. Chem. 77, 2390 (1973).
Q. D. Craft, Ph.D. Thesis, University of Tennessee, 1975.
M. H. Lietzke, ORNL Report No. 3259, Oak Ridge National Laboratory, Oak Ridge, Tennessee, April 1962.
P. Debye and E. Hückel,Phys. Z. 24, 185, 334 (1923);25, 97 (1924).
H. S. Harned and B. B. Owen,The Physical Chemistry of Electrolyte Solution, 2nd ed. (Reinhold Publishing Corporation, New York, 1954), p. 379.
E. Hückel,Phys. Z. 26, 93 (1925).
R. W. Stoughton and M. H. Lietzke,J. Chem. Eng. Data 10, 254 (1965).
F. J. Millero,J. Phys. Chem. 74, 356 (1970).
Y. S. Choi and O. D. Bonner,Z. Phys. Chem. 87, 188 (1973).
G. S. Kell,J. Chem. Eng. Data 12, 66 (1967).
G. A. Vidulich, D. F. Evans, and R. L. Kay,J. Phys. Chem. 71, 656 (1967).
C. M. Criss and J. W. Cobble,J. Am. Chem. Soc. 83, 3223 (1961).
E. A. Gulbransen and A. L. Robinson,J. Am. Chem. Soc.,56, 2637 (1934).
V. B. Parker,Thermal Properties of Aqueous Uni-Univalent Electrolytes, NSRDSNBS2 (1965).
D. D. Ensor and H. L. Anderson,J. Chem. Eng. Data 18, 205 (1973).
C. E. Vanderzee and L. J. Gier,J. Chem. Thermodyn. 6, 441 (1974).
Q. D. Craft and W. A. Van Hook,J. Solution Chem. 4, 923 (1975).
T. F. Young and J. S. Machin,J. Am. Chem. Soc. 58, 2252 (1936).
I. M. Klotz,Chemical Thermodynamics Prentice-Hall, Inc., Englewood Cliffs, New Jersey, 1957) p. 218.
D. H. Davies and G. C. Benson,Can. J. Chem. 43, 3100 (1965).
E. Lange and W. Martin,Z. Phys. Chem. A180, 233 (1937).
G. N. Lewis, M. Randall, K. S. Pitzer, and L. Brewer,Thermodynamics, 2nd ed. (McGraw-Hill, New York, 1961), pp. 375, 382.
C. V. Krishnan and H. L. Friedman,J. Phys. Chem. 74, 2356 (1970).
Author information
Authors and Affiliations
Additional information
Abstracted in part from a thesis submitted by Q.D.C. in 1975 to the Chemistry Department, University of Tennessee, in partial fulfillment of the requirements for the Ph.D. degree in Chemistry.
Rights and permissions
About this article
Cite this article
Craft, Q.D., Van Hook, W.A. Isotope effects in aqueous systems. V. Partial molal enthalpies of solution of NaCl−H2O−D2O and related systems (5 to 75°C)1 . J Solution Chem 4, 901–922 (1975). https://doi.org/10.1007/BF00645888
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00645888