Abstract
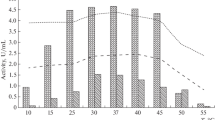
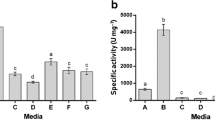
The production of two extracellular proteases, an endopeptidase and an aminopeptidase, by the marine bacteriumVibrio SA1 was studied in batch cultures. The production of the proteases was induced during growth of the organism in peptone media and by several amino acids during growth in minimal media. It was repressed by easily metabolisable carbon compounds such as glucose, lactate and succinate during growth in peptone media. During growth in a lactate basal medium, phenylalanine was one of the best inducers and this amino acid was therefore used in further experiments. That lactate dit not repress the synthesis of the proteases during growth in the lactate basal medium supplemented with 2mm phenylalanine as an inducer, appeared to be a consequence of the low iron content of this medium. Growth curves ofVibrio SA1 on such media showed a period of linear growth during which protease production was observed. When the iron concentration was made sufficiently high to prevent linear growth, the synthesis of the proteases remained repressed. Apparently by imposing an iron limitation on the organism, catabolite repression by lactate was relieved. Similarly, when growth was limited by very low values of the dissolved oxygen tension in the medium, a high rate of protease synthesis was found which was immediately repressed when the oxygen limitation was released. The results indicate that the growth rate and/or a factor associated with the energy metabolism play a role in the regulation of the synthesis of the enzymes.
Similar content being viewed by others
References
Baumann, P., Baumann, L andMandel, M. 1971. Taxonomy of marine bacteria: the genusBeneckea. — J. Bacteriol.107: 268–294.
Boethling, R. S. 1975. Regulation of extracellular protease secretion inPseudomonas maltophilia. — J. Bacteriol.123: 954–961.
Buchanan, B. B. andGibbons, N. E. 1974. Bergey's Manual of Determinative Bacteriology, 8th ed. — Williams and Wilkins, Baltimore.
Daatselaar, M. C. C. andHarder, W. 1974. Some aspects of the regulation of the production of extracellular proteolytic enzymes by a marine bacterium. — Arch. Microbiol.101: 21–34.
De Ley, J. 1970. Reexamination of the association between melting point, buoyant density, and chemical base composition of deoxyribonucleic acid. — J. Bacteriol.101: 738–754.
De Lumen, B. O. andTappel, A. L. 1970. Fluorescein-hemoglobin as a substrate for cathepsin D and other proteases. — Anal. Biochem.36: 22–39.
Glenn, A. R. 1976. Production of extracellular proteins by bacteria. — Annu. Rev. Microbiol.30: 41–62.
Glenn, A. R., Both, G. W., McInnes, J. L., May, B. K. andElliott, W. H. 1973. Dynamic state of the messenger RNA pool specific for extracellular protease inBacillus amyloliquefaciens: its relevance to the mechanism of enzyme secretion. — J. Mol. Biol.73: 221–230.
Hohorst, H. J. 1963.l (+)-lactate: Determination with lactic dehydrogenase and DPN, p. 266–270.In H. U. Bergmeyer, (ed.), Methods of enzymatic analysis. — Verlag Chemie GmbH, Weinheim.
Holding, A. J. andCollee, J. G. 1971. Routine biochemical tests, p. 1–32.In J. R. Norris and D. W. Ribbons, (eds.), Methods in Microbiology, Vol. 6A — Academic Press, London and New York.
Hugh, R. andLeifson, E. 1953. The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram-negative bacteria. — J. Bacteriol.66: 24–26.
Lankford, C. E. 1973. Bacterial assimilation of iron. — C.R.C. Crit. Rev. Microbiol.2: 273–331.
Litchfield, C. D. andPrescott, J. M. 1970a. Regulation of proteolytic enzyme production byAeromonas proteolytica. I. Extracellular endopeptidase. — Can. J. Microbiol.16: 17–22.
Litchfield, C. D. andPrescott, J. M. 1970b. Regulation of proteolytic enzyme production byAromonas proteolytica. II. Extracellular aminopeptidase. — Can. J. Microbiol.16: 23–27.
Mandel, M., Leadbetter, E. R., Pfennig, N. andTrüper, H. G. 1971. Deoxyribonucleic acid base compositions of phototrophic bacteria. — Intern. J. Systematic Bacteriol.21: 222–230.
Marmur, J. andDoty, P. 1962. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. — J. Mol. Biol.5: 109–118.
May, B. K. andElliot, W. H. 1968. Characteristics of extracellular protease formation byBacillus subtilis and its control by amino acid repression. — Biochim. Biophys. Acta157: 607–615.
Merkel, J. R. 1972. Influence of salts on the vibriostatic action of 2,4-diamino-6,7-diisopropylpteridine. — Arch. Microbiol.81: 379–382.
Okinaka, R. T. andDobrogosz, W. J. 1967. Catabolite repression and pyruvate metabolism inEscherichia coli. — J. Bacteriol.93: 1644–1650.
Reichelt, J. L., Baumann, P. andBaumann, L. 1976. Study of genetic relationships among marine species of the generaBeneckea andPhotobacterium by means of in vitro DNA/DNA hybridization. — Arch. Microbiol.110: 101–120.
Skerman, V. B. D. 1967 (ed.). A guide to the identification of the genera of bacteria, 2nd ed. —Williams and Wilkins, Baltimore.
Stinson, M. W. andMerrick, J. M. 1974. Extracellular enzyme secretion byPseudomonas lemoignei. — J. Bacteriol.119: 152–161.
Tanaka, S. andIuchi, S. 1971. Induction and repression of an extracellular proteinase inVibrio parahaemolyticus. — Biken J.14: 81–96.
Tuppy, H., Wiesbauer, U. undWintersberger, E. 1962. Aminosäure-p-nitro-anilide als Substrate für Aminopeptidasen und andere proteolytische Fermente. — Z. Physiol. Chem.329: 278–288.
Wiersma, M. andHarder, W. 1978. A continuous culture study of the regulation of extracellular protease production inVibrio SA1. — Antonie van Leeuwenhoek44: 141–155.
Author information
Authors and Affiliations
Additional information
This study was supported by the Foundation for Fundamental Biological Research (BION), which is subsidised by the Netherlands Organization for the Advancement of Pure Research (ZWO).
Rights and permissions
About this article
Cite this article
Wiersma, M., Hansen, T.A. & Harder, W. Effect of environmental conditions on the production of two extracellular proteolytic enzymes byVibrio SA1. Antonie van Leeuwenhoek 44, 129–140 (1978). https://doi.org/10.1007/BF00643216
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00643216