Abstract
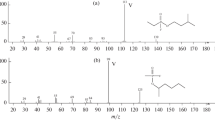
The secondary emission (SE) spectra of six glycosides of medicagenic acid (MA) and of hederagenin have been studied. The (M + H)+ ions were observed in the secondary ion spectra (LSIMS) of mono- and biosides of MA and also of a triand a tetraoside of hederagenin in a glycerol matrix. The (M + Na)+ ions were recorded in the spectra of tri- and tetraosides of MA. In the majority of cases processes involving the successive splitting out of the carbohydrate units were revealed. Positive- and negative-ion spectra have been obtained on the ionization by bombardment with fast atoms — FAB “+” and FAB “−” — of a trioside and a tetraoside of MA. The FAB “−” spectra are the most characteristic: They show intense peaks of the (M - H)− ions and of the products of the sequential elimination of the sugar residues.
Similar content being viewed by others
Literature cited
A. É. Timbekova and N. K. Abubakirov, Khim. Prir. Soedin., 451 (1984).
A. É. Timbekova and N. K. Abubakirov, Khim. Prir. Soedin., 805 (1985).
T. Nagao, R. Tanaka, H. Okabe, and T. Yamauchi, Chem. Pharm. Bull.,38, No. 2, 378 (1990).
V. U. Ahmad, S. Perveen, and S. Bano, Phytochemistry,29, No. 10, 3287 (1990).
H. Kambara and S. Hishida, Anal. Chem.,53, No. 14, 2340 (1981).
A. L. Burlingame, J. O. Whitney, and D. H. Russell, Anal. Chem.,56, 417R (1984).
G. A. Tolstikov, Outlines of the History of Mass Spectrometry [in Russian], Bashkir Scientific Center, Urals Branch, Russian Academy of Sciences, Ufa (1988), p. 158.
N. M. Fales, E. A. Sokoloski, L. K. Pannell, Quan-Long Pu, D. L. Klayman, Ai J. Lin, A. Brossi, and J. A. Kelley, Anal. Chem.,62, 2494 (1990).
A. É. Timbekova and N. K. Abubakirov, Khim. Prir. Soedin., 610 (1986); A. É. Timbekova and N. K. Abubakirov, Khim. Prir. Soedin., 607 (1986); A. É. Timbekova, M. F. Larin, M. R. Yagudaev, and N. K. Abubakirov, Khim. Prir. Soedin., 673 (1989).
R. K. Boyd and J. H. Beynon, Org. Mass Spectrom.,12, 136 (1977).
Yu. M. Mil'grom, V. L. Sadovskaya, Ya. V. Rashkes, and Yu. S. Vollerner, Khim. Prir. Soedin., 53 (1991).
Additional information
Institute of the Chemistry of Plant Substances, Uzbekstan Academy of Sciences, Tashkent. Translated from Khimiya Prirodnykh Soedinenii, No. 1, pp. 98–102, January–February, 1992.
Rights and permissions
About this article
Cite this article
Mil'grom, Y.M., Rashkes, Y.V. & Timbekova, A.É. Secondary-emission mass spectrometry of hederagenin glycosides and medicagenic acid glycosides. Chem Nat Compd 28, 82–85 (1992). https://doi.org/10.1007/BF00629799
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00629799