Summary
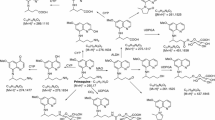
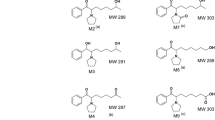
Healthy male volunteers (n=13) took a single oral dose of 60.3 mg of methoxyphenamine HCl with and without prior administration of either quinidine (250 mg as bisulphate salt) or its diastereomer quinine (300 mg as sulphate salt). Methoxyphenamine and its N-desmethyl, O-desmethyl and aromatic 5-hydroxy metabolites were quantified in the 0–32 h urine.
The oxidative routes of methoxyphenamine metabolism which had been previously shown to involve debrisoquine 4-hydroxylase, namely O-demethylation and 5-hydroxylation were both significantly inhibited by quinidine in the 12 extensive metabolizers. The inhibition was selective in that N-demethylation which does not involve this isozyme was not affected by quinidine. In all but one of these volunteers the methoxyphenamine/O-desmethylmethoxyphenamine ratio changed such that extensive metabolizers could be classified as poor metabolizers due to quinidine pretreatment. No marked change occurred in the renal excretion of methoxyphenamine and its three metabolites either in the extensive metabolizers because of quinine pretreatment or in the poor metabolizer because of treatment with either quinidine or quinine.
Thus in the extensive metabolizer phenotype it was demonstrated in one study that enzyme inhibition of quinidine was selective in terms of the metabolic pathways inhibited as well as stereoselective with respect to the inhibitor.
Similar content being viewed by others
References
Brøsen K (1990) Recent developments in hepatic drug oxidation, implications for clinical pharmacokinetics. Clin Pharmacokinet 18: 220–239
Kalow W (1987) Genetic variation in the human hepatic cytochrome P-450 system. Eur J Clin Pharmacol 31: 633–641
Roy SD, Hawes EM, McKay G, Korchinski ED, Midha KK (1985) Metabolism of methoxyphenamine in extensive and poor metabolizers of debrisoquin. Clin Pharmacol Ther 38: 128–133
Speirs CJ, Murray S, Boobis AR, Seddon CE, Davies DS (1986) Quinidine and the identification of drugs whose elimination is impaired in subjects classified as poor metabolizers of debrisoquine. Br J Clin Pharmacol 22: 739–743
Boobis AR, Edwards RJ, Singleton A, Sesardic D, Murray B, Speirs CJ, Murray S, Kobayashi S, Davies DS (1988) The contribution of polymorphic isozymes of cytochrome P450 to the pharmacokinetics and toxicity of foreign compounds in man. In: Miners JO, Birkett DJ, Drew R, May BK, McManus ME (eds) Microsomes and drug oxidations, Proceedings of the 7th International Symposium. Taylor and Francis, London
Nielsen MD, Brøsen K, Gram LF (1990) A dose-effect study of the in vivo inhibitory effect of quinidine on sparteine oxidation in man. Br J Clin Pharmacol 29: 299–304
Otton SV, Inaba T, Kalow W (1984) Competitive inhibition of sparteine oxidation in human liver by β-adrenoceptor antagonists and other cardiovascular drugs. Life Sci 34: 73–80
Inaba T, Jurima M, Mahon WA, Kalow W (1985) In vitro inhibition studies of two isozymes of human liver cytochrome P-450: mephenytoin p-hydroxylase and sparteine monooxygenase. Drug Metab Dispos 13: 443–448
Von Bahr C, Spina E, Birgersson C, Ericsson Ö, Göransson M, Henthorn T, Sjöqvist F (1985) Inhibition of desmethylimipramine 2-hydroxylation by drugs in human liver microsomes. Biochem Pharmacol 34: 2501–2505
Steiner E, Dumont E, Spina E, Dahlqvist R (1988) Inhibition of desipramine 2-hydroxylation by quinidine and quinine. Clin Pharmacol Ther 43: 577–581
Inaba T, Tyndale RE, Mahon WA (1986) Quinidine: potent inhibition of sparteine and debrisoquine oxidation in vivo. Br J Clin Pharmacol 22: 199–200
Notterman DA, Drayer DE, Metakis L, Reidenberg MM (1986) Stereoselective renal tubular secretion of quinidine and quinine. Clin Pharmacol Ther 40: 511–517
Hedman A, Angelin B, Arvidsson A, Dahlqvist R, Nilsson B (1990) Interactions in the renal and biliary elimination of digoxin: stereoselective difference between quinine and quinidine. Clin Pharmacol Ther 47: 20–26
McKay G, Cooper JK, Hawes EM, Roy SD, Midha KK (1983) Identification of new secondary metabolites of methoxyphenamine in man. Xenobiotica 13: 257–264
Muralidharan G, Midha KK, McKay G, Hawes EM, Inaba T (1989) Selective in vivo inhibition by quinidine of methoxyphenamine oxidation in rat models of human debrisoquine polymorphism. Xenobiotica 19: 189–197
Roy SD, Hawes EM, Midha KK (1987) Influence of urinary pH on the disposition of methoxyphenamine and three metabolites in humans. J Pharm Sci 76: 427–432
Brøsen K, Davidsen F, Gram LF (1990) Quinidine kinetics after a single oral dose in relation to the sparteine oxidation polymorphism in man. Br J Clin Pharmacol 29: 248–253
Brøsen K, Gram LF (1989) Quinidine inhibits the 2-hydroxylation of imipramine and desipramine but not the demethylation of imipramine. Eur J Clin Pharmacol 37: 155–160
Funck-Brentano C, Turgeon J, Woosley RL, Roden DM (1989) Effect of low dose quinidine on encainide pharmacokinetics and pharmacodynamics. Influence of genetic polymorphism. J Pharmacol Exp Ther 249: 134–142
Leemann T, Dayer P, Meyer UA (1986) Single-dose quinidine treatment inhibits metoprolol oxidation in extensive metabolizers. Eur J Clin Pharmacol 29: 739–741
Brinn R, Brøsen K, Gram LF, Haghfelt T, Otton SV (1986) Sparteine oxidation is practically abolished in quinidine-treated patients. Br J Clin Pharmacol 22: 194–197
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Muralidharan, G., Hawes, E.M., McKay, G. et al. Quinidine but not quinine inhibits in man the oxidative metabolic routes of methoxyphenamine which involve debrisoquine 4-hydroxylase. Eur J Clin Pharmacol 41, 471–474 (1991). https://doi.org/10.1007/BF00626372
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00626372