Summary
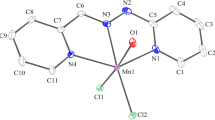
The preparation of transition metal complexes containing the sterically hindered ligand, bis(3,5-dimethylpyrazolyl)methane (LL) is described. Compounds of formula M(LL)X2 (M = CoII, NiII or ZnII and X = Cl− or Br−) or M(LL)2X2 (M = MnII, FeII, CoII, NiII, CuII, ZnII or CdII and X = ClO −4 ; M = CoII, NiII, CuII or ZnII and X = NO −3 ; M = NiII or CuII and X = Cl− or Br−) have been isolated. In addition, an apparently trimeric Cu3(LL)4Cl6 · EtOH compound is reported. For Ni(LL)Cl2 a five-coordinated chloro-bridged dimer is found. The perchlorato compounds, M(LL)2(ClO4)2, appear to have one bidentate ClO −4 and one ionic ClO −4 group. The M(LL)2 species appears to occur either in octahedral geometry, leaving twocis-positions free, or in a tetrahedral geometry without space for other ligands, and probably also in a five-coordinate geometry with one free ligand position.
Structural conclusions are drawn from i.r., far-i.r. and ligand-field spectra, x-ray powder patterns, magnetic susceptibility data, e.s.r. spectra and conductivity data.
Similar content being viewed by others
References
N. A. Daugherty and J. H. Swisher,Inorg. Chem., 7, 1651 (1968).
S. Trofimenko,Chem. Rev., 72, 497 (1972).
J. Reedijk,Rec. Trav. Chim., 88, 1451 (1968).
K. Fukushima, A. Kobayashi, T. Miyamoto and Y. Sasaki,Bull. Chem. Soc. Japan, 49, 143 (1976).
J. Reedijk, J. C. Jansen, H. van Koningsveld and C. G. van Kralingen,Inorg. Chem., 17, 1990 (1978).
C. W. Reimann, A. D. Mighell and F. A. Mauer,Acta Crystallogr., 23, 135 (1967).
J. Reedijk, B. A. Stork-Blaisse and G. C. Verschoor,Inorg. Chem., 10, 2594 (1971).
S. Trofimenko,J. Am. Chem. Soc., 92, 5118 (1970).
S. Trofimenko,J. Am. Chem. Soc., 88, 1842 (1966).
S. Trofimenko,Acc. Chem. Res., 4, 17 (1971).
F. A. Cotton, C. A. Murillo and B. R. Stults,Inorg. Chim. Acta, 22, 75 (1977).
J. Reedijk and J. Verbiest,Transition Met. Chem., 3, 51 (1978).
A. B. P. Lever,J. Chem. Educ., 45, 711 (1968).
M. A. Guichelaar, J. A. M. van Hest and J. Reedijk,Delft Progr. Rep., 2, 51 (1976).
J. C. Jansen, H. van Koningsveld, J. A. C. van Ooyen and J. Reedijk, to be published.
E. J. Pleau and G. F. Kokoszka,J. Chem. Soc. Faraday Trans. II, 69, 355 (1973).
A. B. P. Lever,Inorganic Electronic Spectroscopy, Elsevier, New York, 1968.
B. J. Hathaway and D. E. Billing,Coord. Chem. Rev., 5, 143 (1970).
W. J. Geary,Coord. Chem. Rev., 7, 81 (1971).
K. Nakamoto,Infrared and Roman Spectra of Inorganic and Coordination Compounds, Wiley, New York, 1978.
M. R. Rosenthal,J. Chem. Educ., 50, 331 (1973).
A. B. P. Lever, E. Mantovani and B. S. Ramaswamy,Can. J. Chem., Chem., 49, 1957 (1971).
S. F. Pavkovic and D. W. Meek,Inorg. Chem., 4, 1091 (1965).
A. E. Wickenden and R. A. Krause,Inorg. Chem., 4, 404 (1965).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reedijk, J., Verbiest, J. Coordination compounds derived from transition metal salts and bis(3,5-dimethylpyrazolyl)methane. Transition Met Chem 4, 239–243 (1979). https://doi.org/10.1007/BF00619177
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00619177