Summary
-
1.
The motor circuits that control telson flexion in the crayfish (Procambarus clarkii) include a curiously arranged subcircuit: a premotor ‘command’ neuron excites a motor neuron via a trisynaptic pathway, but also inhibits (and prevents firing of) the motor neuron via a shorter latency pathway (Kramer et al. 1981a). The premotor and motor neurons in this circuit have been previously identified (Kramer et al. 1981a; Dumont and Wine 1985a, b; see Fig. 1). We have now identified a local interneuron that inhibits the motor neurons.
-
2.
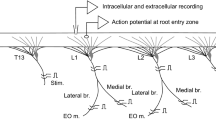
The cell we studied is called the ‘C’ cell because of its distinctive structure (Figs. 2, 3). A single pair of bilaterally homologous C-cells was found in the last (6th) abdominal ganglion. The C-cells are invariably dye coupled to one another following injections of lucifer yellow into either one of them, and are frequently dye coupled to smaller axons in the 2nd, 3rd, and 6th nerves. In addition, some of the extensive branches of the C-cell extend out into the 6th nerve, where they are in close proximity to the axons of the motor neurons they inhibit (Fig. 3).
-
3.
Two kinds of evidence established that the C-cell directly inhibits the motor neurons. First, when simultaneous recordings were made from the C-cell and the motor neurons, spikes in the C-cell, no matter how evoked, were invariably followed, within 1.5 ms, by depolarizing IPSPs in the motor neuron (Fig. 6). Second, when the C-cell was hyperpolarized so that it could not fire, that same IPSP in the motor neuron was abolished (Fig. 6).
-
4.
The inhibitory pathway to the motor neurons must be fired at short latency in order to prevent firing caused by the trisynaptic excitatory input (Fig. 1). The C-cells were fired at short latency (<3 ms) by impulses in either of the escape command cells (Fig. 4), and at even shorter latency by impulses in the Segmental Giant of the 6th ganglion (SG6) (Fig. 5). It has been established elsewhere that the SGs are a major output pathway of the escape command cells; our results suggest that they may be the pathway for commandevoked firing of the C-cell.
-
5.
The C-cells are also excited by two descending, non-giant, flexion premotor neurons, called I2 and I3 (Fig. 5). The EPSPs from a single I2 or I3 impulse were subthreshold, but temporal and spatial summation of EPSPs from the non-giant pathway sometimes fired the C-cells.
-
6.
The IPSP produced by a single C-cell impulse was only a small proportion (<15%) of the total IPSP produced by command cell firing. However, each motor neuron received IPSPs from both C-cells, and the C-cells typically fired multiple spikes. The remainder of the compound IPSP produced by the command cells consisted of summated IPSPs from other inhibitory interneurons, including interganglionic ones (Dumont and Wine 1985a, b).
-
7.
The identification of a major local inhibitory neuron should help in the interpretation of the functional significance of this unusual subcircuit.
Similar content being viewed by others
Abbreviations
- CDI :
-
corollary discharge interneuron
- FF :
-
fast flexor motor neuron
- LG :
-
lateral giant neuron
- MG :
-
medial giant neuron
- MoG :
-
motor giant neuron
- SG :
-
segmental giant neuron
References
Burrows M, Siegler MVS (1978) Graded synaptic transmission between local interneurones and motor neurones in the metathoracic ganglion of the locust. J Physiol 285:231–255
Dumont JPC, Wine JJ (1985a) The telson flexor neuromuscular system of the crayfish. I. Homology with the fast flexor system. J Exp Biol (Submitted)
Dumont JPC, Wine JJ (1985 b) The telson flexor neuromuscular system of the crayfish. II. Segment-specific differences in connectivity between premotor neurones and efferents. J Exp Biol (Submitted)
Dumont JPC, Wine JJ (1985c) The telson flexor neuromuscular system of the crayfish. III. The role of inhibition shaping a stereotyped behaviour pattern. J Exp Biol (Submitted)
Furshpan EJ, Potter DD (1959) Slow postsynaptic potentials recorded from the giant motor fibre of the crayfish. J Physiol 145:326–335
Hanker JS, Yates PE, Metz CB, Rustioni A (1977) A new specific, sensitive and noncarcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J 9:789–792
Kennedy D, Mellon Jr D (1964) Synaptic activation and receptive fields in crayfish interneurons. Comp Biochem Physiol 13:275–300
Kirk MD (1985) Presynaptic inhibition in the crayfish CNS: pathways and synaptic mechanisms. J Neurophysiol 54:1305–1325
Kirk MD, Wine JJ (1984) Identified interneurons produce both primary afferent depolarization and presynaptic inhibition. Science 225:854–856
Kramer AP, Krasne FB (1984) Crayfish escape behavior: production of tailflips without giant fiber activity. J Neurophysiol 52:189–211
Kramer AP, Krasne FB, Bellman KL (1981a) Different command neurons select different outputs from a shared premotor interneuron of crayfish tailflip circuitry. Science 214:810–812
Kramer AP, Krasne FB, Wine JJ (1981b) Interneurons between giant axons and motoneurons in crayfish escape circuitry. J Neurophysiol 45:550–573
Miller LA, Hagiwara G, Wine JJ (1985) Segmental differences in pathways between crayfish giant axons and fast flexor motoneurons. J Neurophysiol 53:252–265
Mittenthal JE, Wine JJ (1973) Connectivity patterns of crayfish giant interneurons: visualization of synaptic regions with cobalt dye. Science 179:182–184
Ochi R (1969) Ionic mechanism of the inhibitory postsynaptic potential of crayfish giant motor fiber. Eur J Physiol 311:131–143
Paul DH, Mulloney B (1985) Local interneurons in the swimmeret system of the crayfish. J Comp Physiol A 156:489–502
Pearson K (1979) Local neurons and local interactions in the nervous systems of invertebrates. In: Schmitt FO, Worden FG (eds) The neurosciences fourth study program. MIT Press, Cambridge, pp 145–157
Popov AV, Markovich AM, Andjan AS (1978) Auditory interneurons in the prothoracic ganglion of the cricket,Gryllus bimaculatus DeGeer. I. The large segmental auditory neuron (LSAN). J Comp Physiol 126:183–192
Reichert H, Plummer MR, Hagiwara G, Roth RL, Wine JJ (1982) Local interneurons in the terminal abdominal ganglion of the crayfish. J Comp Physiol 149:145–162
Reichert H, Plummer MR, Wine JJ (1983) Identified nonspiking local interneurons mediate nonrecurrent, lateral inhibition of crayfish mechanosensory interneurons. J Comp Physiol 151:261–276
Roberts AM, Krasne FB, Hagiwara G, Wine JJ, Kramer AP (1982) Segmental Giant: evidence for a driver neuron interposed between command and motor neurons in the crayfish escape system. J Neurophysiol 47:761–781
Rowell CHF, Pearson KG (1983) Ocellar input to the flight motor system of the locust: structure and function. J Exp Biol 103:265–288
Schrameck JE (1970) Crayfish swimming: Alternation output and giant fiber activity. Science 169:698–700
Siegler MVS, Burrows M (1983) Spiking local interneurons as primary integrators of mechanosensory information in the locust. J Neurophysiol 50:1281–1295
Siegler MVS, Burrows M (1984) The morphology of two groups of spiking local interneurons in the metathoracic ganglion of the locust. J Comp Neurol 224:463–482
Sigvardt KA, Hagiwara G, Wine JJ (1982) Mechanosensory integration in the crayfish abdominal nervous system: structural and physiological differences between interneurons with single and multiple spike initiating sites. J Comp Physiol 148:143–157
Stewart WW (1978) Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell 14:741–759
Takahata M, Nagayama T, Hisada M (1981) Physiological and morphological characterization of anaxonic non-spiking interneurons in the crayfish motor control system. Brain Res 226:309–314
Tautz T, Müller-Tautz R (1983) Antennal neuropile in the brain of the crayfish: morphology of neurons. J Comp Neurol 218:415–425
Van Harreveld A (1936) A physiological solution for fresh water Crustacea. Proc Soc Exp Biol Med 34:428–432
Watson AHD, Burrows M (1983) The morphology, ultrastructure, and distribution of synapses on an intersegmental interneurone of the locust. J Comp Neurol 214:154–169
Wiersma CAG (1938) Function of the giant fibers of the central nervous system of the crayfish. Proc Soc Exp Biol Med 38:661–662
Wine JJ (1977) Neuronal organization of crayfish escape behavior: inhibition of giant motoneuron via a disynaptic pathway from other motoneurons. J Neurophysiol 40:1078–1097
Wine JJ (1984) The structural basis of an innate behavioural pattern. J Exp Biol 112:283–319
Wine JJ, Krasne FB (1972) The organization of escape behaviour in the crayfish. J Exp Biol 56:1–18
Wine JJ, Krasne FB (1982) The cellular organization of crayfish escape behavior. In: Bliss DE (ed) The biology of Crustacea, vol 4, Academic Press, New York, pp 241–292
Wohlers DW, Huber F (1978) Intracellular recording and staining of cricket auditory interneurons (Gryllus campestris L.,Gryllus bimaculatus DeGeer). J Comp Physiol 127:11–28
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kirk, M.D., Dumont, J.P.C. & Wine, J.J. Local inhibitor of the crayfish telson-flexor motor giant neurons: morphology and physiology. J. Comp. Physiol. 158, 69–79 (1986). https://doi.org/10.1007/BF00614521
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00614521