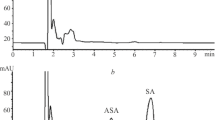
Summary
An aqueous solution of35S-ampicillin and polyethylene glycol (PEG 4000, unabsorbable marker) was administered orally to 6 healthy subjects with gastro-intestinal tubes. The minimal absorption of radioactive material, as shown by urinary excretion of label, differed markedly between subjects and averaged 44% (25 – 67). As the mean cumulative uptake in the proximal jejunum was 31% (24 – 45), it appeared that some of the radioactivity had been taken up in more distal parts of the gastrointestinal tract. Chromatographic fractionation of gastrointestinal aspirates indicated that more than 95% of the label usually remained attached to the parent compound. The corresponding figure for radioactive material recovered from urine was about 80–85%. A similar degree of decomposition was also found for label excreted in the urine of an additional subject who received35S-ampicillin intravenously. In this experiment the urine contained 87% of the administered radioactivity.
Similar content being viewed by others
References
Acocella, G., Mattiussi, R., Nicolis, F.B., Pallanza, R., Teuconi, L.T.: Biliary excretion of antibiotics in man. Gut9, 536–545 (1968)
Anton, A.H.: A drug-induced change in the distribution and renal excretion of sulfonamides. J. Pharmacol. exp. Ther.134, 291–303 (1961)
Ayliffe, G.A.J., Davies, A.: Ampicillin levels in human bile. Brit. J. Pharmacol.24, 189–193 (1965)
Brauer, R.W., Pessotti, R.L.: The removal of bromsulphthalein from blood plasma by the liver of the rat. J. Pharmacol. exp. Ther.97, 358–370 (1949)
Bray, G.A.: A simple efficient liquid scintillator for counting aqueous solutions in a liquid scintillation counter. Analyt. Biochem.1, 279–285 (1960)
Brumfitt, W., Franklin, I., Hayer, L.et al.: Treatment of urinary tract infections with pivampicillin. Scand. J. infect. Dis.5, 59–65 (1973)
Cahn, M.M., Levy, E.J.: Ampicillin potassium: A new salt. Studies on absorption and administration. Hospital (Rio)76, 227–236 (1969)
Cole, M., Kenig, M.D., Hewitt, V.A.: Metabolism of penicillins to penicilloic acids and 6-aminopenicillanic acid and its significance in assessing penicillin absorption. Antimicrob. Agents Chemother.3, 463–468 (1973)
Doyle, F.P., Nayler, J.H.C., Smith, H., Stove, E.R.: Some novel acid-stable penicillins. Nature (Lond.)191, 1091–1092 (1961)
Floch, M.H.: Recent contributions in intestinal absorption and malabsorption. Amer. J. Clin. Nutr.22, 327–351 (1969).
Foltz, E.L., West, J.W., Breslow, I.H., Wallick, H.: Clinical pharmacology of pivampicillin. Antimicrob. Agents Chemother. 442–454 (1970)
Fordtrand, J.S.: Marker perfusion techniques for measuring intestinal absorption in man. Gastroenterology51, 1089–1093 (1966)
Gloxhuber, C., Offe, H.A., Rauenbusch, E., Scholtan, W., Schmid, J.: Dicloxacillin. Arzneimittel-Forsch.15, 322–330 (1965)
Hellström, K., Rosén, A., Söderlund, L.: The gastrointestinal absorption and the excretion of3H-butylscopolamine (hyoscine butylbromide) in man. Scand. J. Gastroent.5, 585–592 (1970)
Hellström, K., Rosén, A., Swahn, A.: Fate of oral35S-cloxacillin in man. Europ. J. clin. Pharmacol.7, 125–131 (1974a)
Hellström, K., Rosén, A., Swahn, A.: Absorption and decomposition of potassium35S-phenoxy-methylpenicillin. Clin. Pharmacol. Ther.16, 826–833 (1974b)
Hydén, S.: A turbidimetric method for the determination of higher polyethylene glycols in biological materials. Kungliga Lantbrukshögskolans Annaler22, 139–145 (1955)
Jeffay, H., Olubajo, F.O., Jewell, W.R.: Determination of radioactive sulphur in biological materials. Analyt. Chem.32, 306–308 (1960)
Jusko, W.J., Lewis, G.P.: Comparison of ampicillin and hetacillin pharmacokinetics in man. J. pharm. Sci.62, 69–76 (1973)
Kirby, W.M.M., Gordon, R.C., Reagamey, C.: The pharmacology of orally administered amoxicillin and ampicillin. J. infect. Dis.129, (Suppl.) 154–155 (1974)
Klein, J.O., Finland, M.: Ampicillin. Activityin vitro and absorption and excretion in normal young men. Amer. J. med. Sci.245, 544–555 (1963)
Knudsen, E.T., Rolinson, G.N., Stevens, S.: Absorption and excretion of “Penbritin”. Br. med. J.1961 II, 198–200
Kulhanek, M., Tadra, M.: Products of acylase and beta-lactamase hydrolysis of some penicillins. Folia. microbiol. (Praha)13, 340–345 (1968)
Kunin, C.M.: Clinical pharmacology of the new penicillins. I. The importance of serum protein binding in determining antimicrobial activity and concentration in serum. Clin. Pharmacol. Ther.7, 166–179 (1966)
Lode, H., Janisch, P., Küpper, G., Weuta, H.: Comparative clinical pharmacology of three ampicillins and amoxicillin administered orally. J. infect. Dis.129, (Suppl.) 156–168 (1974).
Loo, J.C.K., Foltz, E.L., Wallick, H., Kwan, K.C.: Pharmacokinetics of pivampicillin and ampicillin in man. Clin. Pharmacol. Ther.16, 35–43 (1974)
Lund, B., Mogensen, C., Mølholm-Hansen, J., Kampmann, J., Siersbaek-Nielsen, K.: Ampicillin in portal and peripheral blood and bile after oral administration of ampicillin and pivampicillin. Europ. J. clin. Pharmacol.7, 133–135 (1974)
Modr, Z., Dvoracek, K.: Pharmacokinetics of ampicillin and hetacillin. Rev. Czech. Med.16, 84–95 (1970)
Mortimer, P.R., Mackie, D.B., Haynes, S.: Ampicillin levels in human bile in the presence of biliary tract disease. Brit. med. J.1969 III, 88–91
Murakawa, T., Wakai, Y., Nishida, M.: Chromatographic assay of mixed penicillins, ampicillin and cloxacillin in body fluids. J. Antibiot. (Tokyo)23, 250–251 (1970)
Nishida, M., Matsubara, T., Murakawa, T., Mine, Y., Yokota, Y.: Cefazolin, a new semisynthetic cephalosporin antibiotic. J. Antibiot. (Tokyo)23, 184–194 (1970)
Nishida, M., Murakawa, T., Mine, Y., Fukada, S., Kono, Y., Sueda, Y.: Studies on the formation and activity of the transformation product of ampicillin. J. Antibiot. (Tokyo)24, 641–646 (1971)
Nosslin, B.: The direct diazo reaction of bile pigments in serum. Scand. J. clin. Lab. Invest.12 (suppl. 49) 1–176 (1960)
Notari, R.E.: Pharmacokinetics and molecular modification: Implications in drug design and evaluation. J. pharm. Sci.62, 865–881 (1973)
Sutherland, R., Robinson, O.P.W.: Laboratory and pharmacological studies in man with hetacillin and ampicillin. Brit. med. J.1967 II, 804–808
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Swahn, A. On the absorption and metabolism of35S-ampicillin. Eur J Clin Pharmacol 9, 117–124 (1975). https://doi.org/10.1007/BF00614007
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00614007