Summary
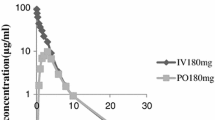
The kinetics of ceftriaxone was investigated in 8 patients without infection, who were receiving continuous ambulatory peritoneal dialysis (CAPD). Ceftriaxone 1 g was injected i.v. and 1 g was given intraperitoneally in the CAPD fluid during a 4-h dwell time. Ceftriaxone was assayed by HPLC. After intravenous administration, the kinetic parameters of ceftriaxone were: plasma t1/2, 12.3 h, total plasma clearance, 14.0 ml/min, volume of distribution at steady state 0.18 l/kg, and peritoneal clearance 0.59 ml/min. Over 72 hours only 5.5% of the dose was eliminated by the peritoneal route. After intraperitoneal administration, ceftriaxone rapidly appeared in serum; the absorption t1/2 was 1.1 h and the mean peak concentration was 38.8 µg/ml. The absorption of ceftriaxone from the peritoneal space was 39%. A single 1.0 g IP dose led to serum and dialysate concentrations of ceftriaxone above the minimum inhibitory concentration for susceptible pathogens for 24 hours.
Similar content being viewed by others
References
Albin HC, Demotes-Mainard FM, Bouchet JL, Vinçon GA, Martin-Dupont C (1985) Pharmacokinetics of intravenous and intraperitoneal cefotaxime in chronic ambulatory peritoneal dialysis. Clin Pharmacol Ther 38: 285–289
Albin HC, Ragnaud JM, Demotes-Mainard F, Vinçon G, Wone C (1986) Pharmacokinetics of intravenous and intraperitoneal moxalactam in chronic ambulatory peritoneal dialysis Eur J Clin Pharmacol 30: 299–302
Arvidsson A, Alvan G, Anvelin B, Borga O, Nord CE (1982) Ceftriaxone renal and biliary excretion and effect on colon microflora. J Antimicrob Chemother 10: 207–215
Arvidsson A, Alvan G, Tranaeus A, Malmborg AS (1985) Pharmacokinetic studies of cefoxitin in continuous ambulatory peritoneal dialysis. Eur J Clin Pharmacol 28: 333–337
Bunke CM, Aronoff GR, Brier HE, Sloan RS, Luft FC (1983) Cefazolin and cephalexin kinetics in continuous ambulatory peritoneal dialysis. Clin Pharmacol Ther 33: 66–72
Cohen D, Appel GB, Scully B, Neu HC (1983) Pharmacokinetics of ceftriaxone in patients with renal failure and in those undergoing hemodialysis. Antimicrob. Agents Chemother 24: 529–532
Gibson TP, Matusals E, Nelson LD, Briges WA (1976) Artificial kidneys and clearance calculations. Clin Pharmacol Ther 20: 720–726
Gomeni R (1984) PHARM — An interactive graphic program for individual and population pharmacokinetic parameter estimation. Comput Biol Med 14: 25–34
Gross ML, Somani P, Ribner BS, Raeader R, Freimer EM, Kiggins TT (1983) Ceftizoxime elimination kinetics in continuous ambulatory peritoneal dialysis. Clin Pharmacol 34: 673–680
Holder JE, Galeazzi RL, Frey B, Rudhardt M, Seiler AJ (1984) Pharmacokinetics of cefoperazone in patients undergoing chronic ambulatory peritoneal dialysis: clinical and pathophysiological implications. Eur J Clin Pharmacol 26: 609–612
McIntosh ME, Smith WGJ, Junor BJR, Forrest G, Brodie MJ (1985) Increased peritoneal permeability in patients with peritonitis undergoing continuous ambulatory peritoneal dialysis. Eur J Clin Pharmacol 28: 187–191
McNamara PJ, Stoeckel K, Ziegler WH (1982) Pharmacokinetics of ceftriaxone following intravenous administration of a 3 g dose. Eur J Clin Pharmacol 22: 71–75
Patel IH, Chen S, Parsonnet M, Hackmann MR, Brooks MA, Konikoff J, Kaplan SA (1981) Pharmacokinetics of ceftriaxone in humans. Antimicrob Agents Chemother 20: 634–641
Patel IH, Sugihara JG, Weinfeld RE, Wong EGC, Siemsen AW, Berman SJ (1984) Ceftriaxone pharmacokinetics in patients with various degrees of renal impairment. Antimicrob Agents Chemother 25: 438–442
Petersen J, Stewart RDM, Catton GRD, Edward N (1985) Pharmacokinetics of intraperitoneal cefotaxime treatment of peritonitis in patients continuous ambulatory peritoneal dialysis. Nephron 40: 79–82
Pickup ME, Bird HA, Lowe JR, Lees L, Wright V (1981) Pharmacokinetic and tolerance study of Ro 13-9904, a new cephalosporin antibiotic. Br J Clin Pharmacol 12: 111–115
Pollock AA, Tee PE, Patel IH, Spicehandler J, Simberkoff MS, Ramai JJ Jr (1982) Pharmacokinetic characteristics of intravenous ceftriaxone in normal adults. Antimicrob Agents Chemother 22: 816–823
Stoeckel K (1981) Pharmacokinetics of Rocephin, a highly active new cephalosporin with an exceptionnally long biological half-life. Chemotherapy 27 [Suppl 1]: 42–46
Stoeckel K, Mc Namara PJ, Hoppe-Seyler G, Blumberg A, Keller E (1983) Single-dose ceftriaxone kinetics in functionally anephric patients. Clin Pharmacol Ther 33: 633–641
Ti TY, Fortin L, Kreeft JH, East DS, Ogilvie RI, Somerville PJ (1984) Kinetic disposition of intravenous ceftriaxone in normal subjects and patients with renal failure on hemodialysis or peritoneal dialysis. Antimicrob Agents Chemother 25: 83–87
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Albin, H., Ragnaud, J.M., Demotes-Mainard, F. et al. Pharmacokinetics of intravenous and intraperitoneal ceftriaxone in chronic ambulatory peritoneal dialysis. Eur J Clin Pharmacol 31, 479–483 (1986). https://doi.org/10.1007/BF00613528
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00613528