Summary
-
1.
Some electrophysiological properties of a multifunctional molluscan muscle, the supralateral radular retractor (SLR), were examined. The resting membrane potential was 62.3±10.5 mV and was dependent upon permeabilities to K+, Na+ and Cl−. Current-voltage relationships for SLR muscle fibres were linear, and the fibres had input resistances of 22.5±5.6 MΩ. Indirect evidence suggested that they were not electrically coupled.
-
2.
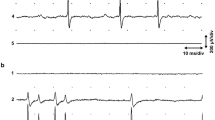
Miniature EJPs occurred spontaneously in the muscle. Action potentials in the identified motoneurones elicited monosynaptic, chemical EJPs in muscle fibres. The majority of fibres were polyneuronally innervated by up to four motor axons. Action potentials did not normally occur in the muscle fibres, and contraction was proportional to the degree of depolarization produced by EJPs.
-
3.
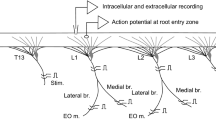
Contractions elicited in the SLRs by the stimulation of motoneurones were bilaterally symmetrical, and were temporally and spatially characteristic of the type of neurone excited. Temporal differences in contraction between motor units were determined mainly by differences in initial EJP amplitude. The relatively small degree of facilitation shown by all motor units had little effect on the speed of contraction. A linear summation model adequately described the growth of facilitation at terminals of one type of motoneurone, but not at those of the other two types of motoneurones examined.
Similar content being viewed by others
Abbreviations
- EDTA :
-
ethylenediaminotetraacetic acid
- EJP :
-
excitatory junctional potential
- L :
-
lateral motoneurone
- M :
-
medial motoneurone
- MEJP :
-
miniature excitatory junctional potential
- P :
-
phasic motoneurone
- R in :
-
input resistance
- SLR :
-
supralateral radular retractor
References
Asada V, Bennett MVL (1971) Experimental alteration of coupling at an electrotonic synapse. J Cell Biol 49:159–172
Atwood HL, Bittner GD (1971) Matching of excitatory and inhibitory inputs to crustacean muscle fibres. J Neurophysiol 134:157–170
Atwood HL, Hoyle G, Smyth T Jr (1965) Mechanical and electrical properties of single innervated crab muscle fibres. J Physiol (Lond) 180:449–482
Banks FW (1978) Central control of the lower extrinsic protractor muscles in the buccal ganglia ofAplysia. Comp Biochem Physiol 61A:267–277
Bennett MVL (1973) Permeability and structure of electrotonic junctions and intercellular movements of tracers. In: Kater SB, Nicholson C (eds) Intracellular staining in neurobiology. Springer, Berlin Heidelberg New York, pp 115–134
Berry MS, Pentreath VW (1976) Criteria for distinguishing between monosynaptic and polysynaptic transmission. Brain Res 105:1–20
Bittner GD, Sewell VL (1976) Facilitation at crayfish neuromuscular junctions. J Comp Physiol 109:287–308
Blankenship JE, Rock MK, Hill J (1977) Physiological properties of the penis retractor muscle ofAplysia. J Neurobiol 8:549–568
Brace RC, Quicke DLJ (1980) Neural control of radular retractor muscles of the pulmonate snail,Planorbarius corneus (L.): functional anatomy and properties of neuromuscular units. JExp Biol 86:115–133
Brace RC, Quicke DLJ (1981) Activity patterns in radular retractor motoneurones of the snail,Planorbarius. J Comp Physiol 142:259–270
Carew TJ, Pinsker H, Rubinson K, Kandel ER (1974) Physiological and biochemical properties of neuromuscular transmission between identified motoneurones and gill muscle inAplysia. J Neurophysiol 37:1020–1040
Cohen JL, Weiss KR, Kupfermann I (1978) Motor control of buccal muscles inAplysia. J Neurophysiol 41:157–180
Cooke JD, Quastel DMJ (1973) Cumulative and persistent effects of nerve terminal depolarization on transmitter release. J Physiol (Lond) 228:407–434
Fatt P, Katz B (1952) Spontaneous subthreshold activity at motor nerve endings. J Physiol (Lond) 117:109–128
Florey E, Kriebel ER (1969) Electrical and mechanical responses of chromatophore muscle fibres of the squid,Loligo opalescens, to nerve stimulation and drugs. Z Vergl Physiol 65:98–130
Hardie J (1978) Electrotonic coupling between supercontracting body-wall muscle fibres and their attachment morphology in the blowfly larva. J Insect Physiol 24:647–655
Heyer CB, Kater SB, Karlsson UL (1973) Neuromuscular systems in molluscs. Am Zool 13:247–270
Hill RB, Licis P (1981) Effect of neurohumours during shutdown of electrogenic sodium pumping in a molluscan muscle. Adv Physiol Sci 22:339–360
Jacklet JW, Rine J (1977) Facilitation at neuromuscular junctions: contribution to habituation and dishabituation of theAplysia gill-withdrawal reflex. Proc Natl Acad Sci USA 74:1267–1271
Jenkinson DH (1957) The nature of the antagonism between calcium and magnesium ions at the neuromuscular junction. J Physiol (Lond) 138:434–444
Kater SB (1974) Feeding inHelisoma trivolvis: the morphological and physiological basis of a fixed action pattern. Am Zool 14:1017–1036
Kater SB, Heyer C, Hegmann JP (1971) Neuromuscular transmission in the gastropod molluscHelisoma trivolvis: identification of motoneurones. Z Vergl Physiol 74:127–139
Katz B, Miledi R (1965) The effect of calcium on acetylcholine release from motor nerve terminals. Proc R Soc Lond B 161:496–503
Kobayashi M, Ando M (1979) Evidence of an electrogenic sodium pump in molluscan radular muscle cells. J Comp Physiol 132:141–147
Linder TM (1974) The accumulative properties of facilitation at crayfish neuromuscular synapses. J Physiol (Lond) 238:223–234
Mallart A, Martin AR (1967) An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol (Lond) 193:679–694
Mellon DeF (1968) Junctional physiology and motor nerve distribution in the fast adductor muscle of the scallop. Science 160:1018–1020
Merickel MB, Eyman ED, Kater SB (1977) Analysis of a network of electrically coupled neurones producing rhythmic activity in the snailHelisoma trivolvis. IEEE Trans Biomed Eng 24:277–287
Orkand PM, Orkand RK (1975) Neuromuscular junctions in the buccal mass ofAplysia: fine structure and electrophysiology of excitatory transmission. J Neurobiol 6:531–548
Penn RD, Lowenstein WR (1966) Uncoupling of a nerve cell membrane junction by calcium-ion removal. Science 157:88–89
Peters M (1979) Motor innervation of the pharynx levator muscle of the snail,Helix pomatia: physiological and histological properties. J Neurobiol 10:137–152
Quicke DLJ (1981) Aspects of the neuronal control of feeding inPlanorbarius corneus (L.). PhD thesis, University of Nottingham
Sawada M, Blankenship JE, McAdoo DJ (1981) Neural control of a molluscan blood vessel, anterior aorta ofAplysia. J Neurophysiol 46:967–986
Schwartz LM (1981) Transegmental electrical coupling between adjacent intersegmental muscles in the tobacco hawkmoth (Manduca sexto). J Insect Physiol 27:727–734
Sorokina ZA (1965) Extra- and intracellular hydrogen ion activity determination in mollusc nerve cell ganglia. J Evol Biochem Physiol 1:343–350
Tareskevich PS, Gibbs D, Schmued L, Orkand RK (1977) Excitatory effects of cholinergic, adrenergic and glutaminergic agonists on a buccal muscle ofAplysia. J Neurobiol 8:325–335
Tattersall JEH (1983) The motor innervation of the supralateral radular retractor muscles ofPlanorbarius corneus (L.). PhD thesis, University of Nottingham
Wilkens LA (1972) Electrophysiological studies on the heart of the bivalve mollusc,Modiolus demissus. I. Ionic basis of the membrane potential. J Exp Biol 56:273–291
Yamaguchi H, Twarog BM (1980) Electrogenic sodium pumping inMytilus smooth muscle. Comp Biochem Physiol 66A:265–270
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tattersall, J.E.H., Brace, R.C. Physiology and motor innervation of the supralateral radular retractor muscles of the pulmonate snail,Planorbarius corneus . J. Comp. Physiol. 160, 115–125 (1987). https://doi.org/10.1007/BF00613447
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00613447