Summary
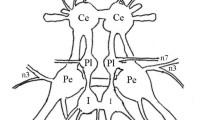
Motor neurons innervating the dorsal longitudinal muscles of a noctuid moth receive synaptic input activated by auditory stimuli. Each ear of a noctuid moth contains two auditory neurons that are sensitive to ultrasound (Fig. 1). The ears function as bat detectors. Five pairs of large motor neurons and three pairs of small motor neurons found in the pterothoracic ganglia innervate the dorsal longitudinal (depressor) muscles of the mesothorax (Figs. 2 to 5). In non-flying preparations the motor neurons receive no oscillatory synaptic input. Synaptic input to a cell resulting from ultrasonic stimulation is consistent and can be either depolarizing or hyperpolarizing (Figs. 6 to 9). Quiescent neurons only rarely fire a spike in response to auditory inputs. Motor neurons in flying preparations receive oscillatory synaptic drive from the flight pattern generator and usually fire a spike for each wingbeat cycle (Figs. 10 to 12). Ultrasonic stimulation can provide augmented synaptic drive causing a neuron to fire two spikes per wingbeat cycle thus increasing flight vigor (Fig. 11). The same stimulus presented on another occasion can also inhibit spiking in the same motor neuron, but the rhythmic drive remains (Fig. 12). Thus, when the flight oscillator is running auditory stimuli can modulate neuronal responses in different ways depending on some unknown state of the nervous system. Sound intensity is the only stimulus parameter essential for activating the auditory pathway to these motor neurons. The intensity must be sufficient to excite two or three auditory neurons. The significance of these responses in relation to avoidance behavior to bats is discussed.
Similar content being viewed by others
References
Bacon JP, Altman JS (1977) A silver intensification method for cobalt-filled neurons in wholemount preparations. Brain Res 138:359–363
Boyan GS (1985) Auditory input to the flight system of the locust. J Comp Physiol A 156:79–91
Boyan GS, Fullard JH (1986) Interneurones responding to sound in the tobacco budworm mothHeliothis virescens (Noctuidae): morphological and physiological characteristics. J Comp Physiol A 158:391–404
Casaday GB, Camhi JM (1976) Metamorphosis of flight motor neurons in the mothManduca sexta. J Comp Physiol 112:143–158
Eggers F (1919) Das thoracale bitympanale Organ einer Gruppe der Lepidoptera Heterocera. Zool Jahrb Abt Anat 41:273–376
Fenton MB, Fullard JH (1981) Moth hearing and the feeding strategies of bats. Am Sci 69:266–275
Goodman CS, O'Shea M, McCaman R, Spitzer NC (1979) Embryonic development of identified neurons: Temporal pattern of morphological and biochemical differentiation. Science 204:1219–1222
Hedwig B, Pearson KG (1984) Patterns of synaptic input to identified flight motoneurons in the locust. J Comp Physiol A 154:745–760
Kondoh Y, Obara Y (1982) Anatomy of motoneurones innervating mesothoracic indirect flight muscles in the silkmoth,Bombyx mori. J Exp Biol 98:23–37
Madsen BM, Miller LM (1981) Neurons innervating some flight muscles in a noctuid moth. Soc Neurosci Abstr 7:950
Miller LA (1983) How insects detect and avoid bats. In: Huber F, Markl H (eds) Neuroethology and behavioral physiology. Springer, Berlin Heidelberg New York, pp 251–266
Miller LA, Olesen J (1979) Avoidance behavior in green lacewings I. Behavior of free-flying green lacewings to hunting bats and ultrasound. J Comp Physiol 131:113–120
Nüesch H (1957) Die Morphologie des Thorax vonTelea polyphemus Cr. (Lepidoptera) II. Nervensystem. Zool Jahrb Abt Anat 75:615–642
Olesen J, Miller LA (1979) Avoidance behavior in green lacewings II. Flight muscle activity. J Comp Physiol 131:121–128
Payne RS, Roeder KD, Wallman J (1966) Directional sensitivity of the ears of noctuid moths. J Exp Biol 44:17–31
Paul DH (1974) Responses to acoustic stimulation of thoracic interneurons in noctuid moths. J Insect Physiol 20:2205–2218
Reichert H, Rowell CHF (1986) Neuronal circuits controlling flight in the locust: how sensory information is processed for motor control. Trends Neurosci 9:281–283
Reichert H, Rowell CHF, Griss C (1985) Course correction circuitry translates feature detection into behavioural action in locusts. Nature 315:142–144
Roeder KD (1962) The behaviour of free-flying moths in the presence of artificial ultrasonic pulses. Anim Behav 10:300–304
Roeder KD (1964) Aspects of the noctuid tympanic nerve response having significance in the avoidance of bats. J Insect Physiol 10:529–546
Roeder KD (1966) Interneurons of the thoracic nerve cord activated by tympanic nerve fibers in noctuid moths. J Insect Physiol 12:1227–1244
Roeder KD (1974a) Acoustic sensory responses and possible bat-evasion tactics of certain moths. Proc Can Soc Zool Annu Meeting, pp 71–78
Roeder KD (1974b) Responses of the less sensitive acoustic sense cells in the tympanic organs of some noctuid and geometrid moths. J Insect Physiol 20:55–66
Roeder KD (1975) Neural factors and evitability in insect behavior. J Exp Zool 194:75–88
Roeder KD, Payne RS (1965) Acoustic orientation of a moth in flight by means of two sense cells. Symp Soc Exp Biol 20:251–272
Roeder KD, Treat AE (1957) Ultrasonic reception by the tympanic organ of noctuid moths. J Exp Zool 134:127–158
Stewart WW (1978) Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell 14:741–759
Surlykke A (1984) Hearing in notodontid moths: a tympanic organ with a single auditory neurone. J Exp Biol 113:323–335
Surlykke A, Miller LA (1982) Central branchings of three sensory axons from a moth ear (Agrotis segetum, Noctuidae). J Insect Physiol 28:357–364
Treat AE (1959) The metathoracic musculature ofCrymodes devastor (Brace) (Noctuidae) with special reference to the tympanic organ. Smithsonian Misc Coll 137:365–377
Tyrer NM, Altman JS (1974) Motor and sensory flight neurones in a locust demonstrated using cobalt chloride. J Comp Neurol 157:117–138
Yinon U, Shulov A, Tsvilich R (1971) Audition in the desert locust: behavioural and neurophysiological studies. J Exp Biol 55:713–725
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Madsen, B.M., Miller, L.A. Auditory input to motor neurons of the dorsal longitudinal flight muscles in a noctuid moth (Barathra brassicae L.). J. Comp. Physiol. 160, 23–31 (1987). https://doi.org/10.1007/BF00613438
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00613438