Abstract
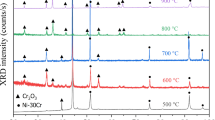
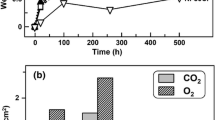
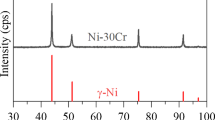
The oxidation of binary Ni-Cr alloys containing 44 and 50 wt. % Cr has been studied over a range of oxygen partial pressures at temperatures between 800 and 1100°C. The effects of cold work, surface preparation, and distribution of the Cr-rich second phase have been studied. The oxidation behavior is complex and cannot be described by a single model. The oxide grows by short-circuit diffusion as well as bulk transport through Cr 2 O 3 scales. The scale-growth mechanism includes extensive metal-oxide separation requiring Cr vapor transport to the scale, compressive stresses within the oxide which result in scale bulging and cracking, and the formation of a second oxide layer which results in voids being incorporated into the scale. Any factor which reduces the oxide grain size, such as cold work, finer distribution of the Cr-rich α phase or reduced oxygen pressure, results in an increased oxidation rate of binary alloys because of an increased number of grain-boundary short-circuit diffusion paths.
Similar content being viewed by others
References
D. M. Johnson, D. P. Whittle, and J. Stringer,Corros. Sci. 15, 721 (1975).
M. R. Wootton and N. Birks,Corros. Sci. 15, 1 (1975).
P. L. Norman and J. D. Harston, “An Evaluation of the Hot Corrosion Resistance of Commercial High Chromium Nickel-Base Alloys for Use in Gas Turbines,” inDeposition and Corrosion in Gas Turbines, A. B. Hart and A. J. B. Cutler, eds. (Wiley, New York, 1973), p. 260.
C. S. Giggins and F. S. Pettit,Trans. Am. Inst. Min. Metall. Pet. Eng. 245, 2495 (1969).
N. Birks and H. Rickert,J. Inst. Met. 91, 308 (1962–63).
J. C. Wood and T. Hodgkiess,J. Electrochem. Soc. 113, 319 (1966).
C. E. Lowell,Oxid. Met. 7, 95 (1973).
C. Wagner,Corros. Sci. 8, 889 (1968).
P. Kofstad and A. Z. Hed,J. Electrochem. Soc. 116, 1542 (1969).
G. C. Wood and D. P. Whittle,J. Electrochem. Soc. 115, 126 (1968).
C. S. Tedmon, Jr.,J. Electrochem: Soc. 113, 766 (1966).
H. Lewis,Metallurgia. 83, 3 (1971).
C. S. Giggins and F. S. Pettit,Metall. Trans. 2, 1071 (1971).
J. Stringer,Oxid. Met. 5, 49 (1972).
G. M. Ecer and G. H. Meier, “Oxidation of High-Chromium Binary Ni-Cr Alloys and Ternary Alloys Containing Ce, Zr, and Ti,” inProperties of High Temperature Alloys, Z. A. Foroulis and F. S. Pettit eds. (The Electrochemical Society Princeton, N.J., 1977), p. 279.
G. M. Ecer and G. H. Meier,Scripta Metall. 1189 (1973).
P. R. Kofstad and A. Z. Hed,J. Electrochem. Soc. 116, 1542 (1969).
W. C. Hagel and A. V. Seybolt,J. Electrochem. Soc. 108, 1146 (1961).
W. C. Hagel,J. Am. Ceram. Soc. 48, 70 (1975).
F. S. Pettit, J. A. Goebel, and G. W. Goward,Corros. Sci. 9, 903 (1969).
G. C. Wood, J. Hodgkiess, and D. P. Whittle,Corros. Sci. 6, 129 (1966).
S. I. Ali and G. C. Wood,J. Inst. Met. 97, 6 (1969).
V. R. Howes,Corros. Sci. 8, 729 (1968).
D. P. Whittle and G. C. Wood,J. Electrochem. Soc. 115, 133 (1968).
E. A. Gulbransen and K. F. Andrew,Trans. Am. Inst. Min. Metall. Pet. Eng. 221, 1247 (1961).
O. Kubaschewski and G. Heymer,Acta Metall. 8, 416 (1960).
H. Davies and W. W. Smeltzer,J. Electrochem. Soc. 121, 543 (1974).
W. C. Hagel,Trans. Am. Soc. Met. 56, 583 (1963).
J. Stringer, A. Z. Hed, G. R. Wallwork, and B. A. Wilcox,Corros. Sci. 12, 625 (1972).
D. Caplan and G. I. Sproule,Oxid. Met. 9, 459 (1975).
D. Caplan, A. Harvey, and M. Cohen,Corros. Sci. 3, 161 (1963).
Author information
Authors and Affiliations
Additional information
This work is based on a portion of a thesis by G. M. Ecer submitted to the University of Pittsburgh in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Metallurgical and Materials Engineering.
Formerly graduate student. Department of Metallurgical and Materials Engineering. University of Pittsburgh.
Rights and permissions
About this article
Cite this article
Ecer, G.M., Meier, G.H. Oxidation of high-chromium Ni-Cr alloys. Oxid Met 13, 119–158 (1979). https://doi.org/10.1007/BF00611976
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00611976