Summary
-
1.
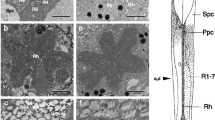
The pupillary response in the compound eye ofManduca sexta results from pigment migration in pigment cells located distal to the retina. We used reflectance photometry to measure the spectral sensitivity of the pupillary response (Figs. 1–4), and compared pupillary action spectra with the ERG spectral sensitivity function and the absorption spectra of retinal visual pigments (Figs. 3–5). Our study was provoked by the report of Hamdorf and Höglund (1981) that the pupillary response of a related moth is maximal in the violet and ultraviolet, but is insensitive to wavelengths greater than 530 nm. Since, in sharp contrast, retinal sensitivity peaks in the green, and drops off at shorter wavelengths, they concluded that the pupillary response is not coupled to retinal excitation, but may result from intrinsic photosensitivity of the pigment cells themselves. Our aim was to test this conclusion by more accurately measuring the spectral response of the pigment cells.
-
2.
The spectral sensitivity of the pupillary response from 460–690 nm matches the ERG action spectrum (Fig. 5), and both correspond to the absorption spectrum of the long wavelength photopigment (P520, λ max = 520 nm) present in the retina (Fig. 3).
-
3.
There is a second peak in pupillary action spectra between 410 and 450 nm that varies from eye to eye in its height relative to the maximum at 520 nm (Fig. 4). The short wavelength peak corresponds to the violet receptors of the retina, whose existence has been inferred from selective adaptation experiments and from the presence of a violet sensitive photopigment (P440, λmax ≅440 nm). A violet peak is not prominent in the dark-adapted ERG spectral sensitivity function (Fig. 5). The variable violet peak in pupillary action spectra may reflect variation in relative input to the pigment cells from the green and violet receptors.
-
4.
We conclude that the pupillary response is probably driven by way of the retinal receptors, rather than depending on a photosensitive system within the pigment cells themselves. As in other insects, the pupillary response inManduca provides the possibility for non-invasively measuring visual function.
Similar content being viewed by others
Abbreviations
- ERG :
-
electroretinogram
- P520 :
-
green sensitive rhodopsin
- P440 :
-
violet sensitive rhodopsin
- P345 :
-
ultraviolet sensitive rhodopsin
References
Bennett RR (1983) Circadian rhythm of visual sensitivity inManduca sexta and its development from an ultradian rhythm. J Comp Physiol 150:165–174
Bennett RR, Brown PK (1983) Properties ofManduca visual pigments in two detergents. Invest Ophthalmol Vis Sci (Suppl) 24:164
Bernard GD (1982) Noninvasive optical techniques for probing insect photoreceptors. In: Packer L (ed) Methods in enzymology, vol 81. Visual pigments and purple membranes I. Academic Press, New York, pp 752–759
Bernard GD, Stavenga DG (1979) Spectral sensitivities of retinular cells measured in intact, living flies by an optical method. J Comp Physiol 134:95–107
Bernard GD, Wehner R (1980) Intracellular optical physiology of the bee's eye. I. Spectral sensitivity. J Comp Physiol 137:193–203
Butler R (1971) The identification and mapping of spectral cell types in the retina ofPeriplaneta americana. Z Vergl Physiol 72:67–80
Dreisig H (1981) The dynamics of pigment migration in insect superposition eyes. J Comp Physiol 143:491–502
Frixione E, Aréchiga H (1981) Ionic dependence of screening pigment migration in crayfish retinal photoreceptors. J Comp Physiol 144:35–43
Griffin DR, Hubbard R, Wald G (1947) The sensitivity of the human eye to infra-red radiation. J Opt Soc Am 37:546–554
Hamdorf K (1979) The physiology of invertebrate visual pigments. In: Autrum H (ed) Handbook of sensory physiology, vol VII/6A. Compararative physiology and evolution of vision in invertebrates: Invertebrate photoreceptors. Springer, Berlin Heidelberg New York, pp 145–224
Hamdorf K, Höglund G (1981) Light induced retinal screening pigment migration independent of visual cell activity. J Comp Physiol 143:305–309
Hamdorf K, Schwemer J (1975) Photoregeneration and the adaptation process in insect photoreceptors. In: Snyder AW, Menzel R (eds) Photoreceptor optics. Springer, Berlin Heidelberg New York, pp 263–289
Höglund G (1963) Glow, sensitivity changes and pigment migration in the compound eye of nocturnal Lepidoptera. Life Sci 2:275–280
Höglund G (1966a) Pigment migration and retinular sensitivity. In: Bernhard CG (ed) Wenner-Gren Center International Symposium Series. Functional organization of the compound eye. Pergamon Press, Oxford New York, pp 77–88
Höglund G (1966b) Pigment migration, light screening and receptor sensitivity in the compound eye of nocturnal Lepidoptera. Acta Physiol Scand (Suppl) 282:1–56
Höglund G, Struwe G (1970) Pigment migration and spectral sensitivity in the compound eye of moths. Z Vergl Physiol 67:229–237
Höglund G, Hamdorf K, Langer H, Paulsen R, Schwemer J (1973) The photopigments in an insect retina. In: Langer H (ed) Biochemistry and physiology of visual pigments. Springer, Berlin Heidelberg New York, pp 167–174
Kirschfeld K, Franceschini N (1969) Ein Mechanismus zur Steuerung des Lichtflusses in den Rhabdomeren des Komplexauges vonMusca. Kybernetik 6:13–22
Kunze P (1979) Apposition and superposition eyes. In: Autrum H (ed) Handbook of sensory physiology, vol VII/6A. Comparative physiology and evolution of vision in invertebrates. Invertebrate photoreceptors. Springer, Berlin Heidelberg New York, pp 441–502
Schwemer J, Langer H (1982) Insect visual pigments. In: Packer L (ed) Methods in enzymology vol 81. Visual pigments and purple membranes I. Academic Press, New York, pp 182–190
Schwemer J, Paulsen R (1973) Three visual pigments inDeilephila elpenor (Lepidoptera, Sphingidae). J Comp Physiol 86:215–229
Stavenga DG (1979) Pseudopupils of compound eyes. In: Autrum H (ed) Handbook of sensory physiology, vol VII/6A. Comparative physiology and evolution of vision in invertebrates: Invertebrate photoreceptors. Springer, Berlin Heidelberg New York, pp 357–439
Welsch B (1977) Ultrastruktur und funktionelle Morphologie der Augen des NachtfaltersDeilephila elpenor (Lepidoptera, Sphingidae). Cytobiologie 14:378–400
White RH, Brown PK, Hurley AK, Bennett RR (1983) Rhodopsins, retinula cell ultrastructure, and receptor potentials in the developing pupal eye of the mothManduca sexta. J Comp Physiol 150:153–163
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
White, R.H., Banister, M.J. & Bennett, R.R. Spectral sensitivity of screening pigment migration in the compound eye ofManduca sexta . J. Comp. Physiol. 153, 59–66 (1983). https://doi.org/10.1007/BF00610343
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00610343