Summary
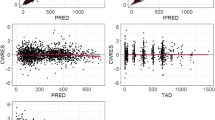
During intermittent melphalan-prednisone therapy the area under the plasma concentration-time curve of melphalan increased by an average of 45% after oral or intravenous administration of the drug in myeloma patients during the initial three courses at six-week intervals.
The rise in melphalan plasma concentrations could not be referred to an alteration in melphalan elimination, metabolism, erythrocyte/plasma partition ratio, or protein binding.
A possible explanation could be that covalent binding sites of melphalan were successively saturated during intermittent treatment, resulting in higher drug concentrations during successive courses of therapy.
Similar content being viewed by others
References
Alberts DS, Chang SY, Chen HSG, Moon TE, Evans TL, Furner RL, Himmelstein K, Gross JF (1979a) Kinetics of intravenous melphalan. Clin Pharmacol Ther 26: 73–80
Alberts DS, Chang SY, Chen HSG, Evans TL, Moon TE (1979b) Oral melphalan kinetics. Clin Pharmacol Ther 26: 737–745
Alexanian R, Haut A, Khan AU, Lane M, McKelvey EM, Migliore PJ, Stuckey WJ, Wilson HE (1969) Treatment for multiple myeloma. J Am Med Ass 208: 1680–1685
Alexanian R, Bonnet J, Gehan E, Haut A, Hewlett H, Lane M, Monto R, Wilson H (1972) Combination chemotherapy for multiple myeloma. Cancer 30: 382–389
Alexanian R, Salmon S, Bonnet J, Gehan E, Haut A, Weick J (1977) Combination therapy for multiple myeloma. Cancer 40: 2765–2771
Ardiet C, Tranchand B, Biron P, Rebattu P, Philip T (1986) Pharmacokinetics of high-dose intravenous melphalan in children and adults with forced diuresis. Cancer Chemother Pharmacol 16: 300–305
Brox L, Birkett L (1979) Pharmacology of intravenous melphalan in patients with multiple myeloma. Cancer Treat Rev 6 [Suppl]: 27–32
Bosanquet AG, Gilby ED (1982) Pharmacokinetics of oral and intravenous melphalan during routine treatment of multiple myeloma. Eur J Cancer Clin Oncol 18: 355–362
Bosanquet AG, Gilby E (1984) Comparison of the fed and fasting states on the absorption of melphalan in multiple myeloma. Cancer Chemother Pharmacol 12: 183–186
Durie BGM, Salmon SE (1975) A clinical staging system for multiple myeloma. Cancer 36: 842–854
Durie BGM, Salmon SE (1982) The current status and future prospects of treatment for multiple myeloma. Clinics in Haematology 11: 181–210
Ecknauer R, Rommel K (1978) Zytostatika und Dünndarm. Klin Wochenschr 56: 579–592
Evans T, Chang SY, Alberts DS, Sipes IG, Brendel K (1982) In vitro degradation of L-phenylalanine mustard (L-PAM). Cancer Chemother Pharmacol 8: 175–178
Furner RF, Brown RK (1980) L-phenylalanine mustard (L-PAM): The first 25 years. Cancer Treat Rep 64: 559–574
Gera S, Musch E, Osterheld HKO, Loos U (1988) Hydrolysis and protein binding of melphalan — their relevance regarding the treatment for multiple myeloma. Cancer Chemother Pharmacol (in press)
Greig NH, Sweeny DJ, Rapoport SI (1987) Melphalan concentration dependent plasma protein binding in healthy humans and rats. Eur J Clin Pharmacol 32: 179–185
Goldenberg G, Lam H, Begleiter A (1979) Active carrier-mediated transport of melphalan by two amino acid transport systems in LPC-1 plasmacytoma cells in vitro. J Biol Chem 254: 1057–1064
Gouyette A, Hartmann O, Pico JL (1986) Pharmacokinetics of high-dose melphalan in children and adults. Cancer Chemother Pharmacol 16: 184–189
Kyle RA (1985) Multiple myeloma: Current therapy and a glimpse of the future. Scand J Haematol 35: 38–47
Loos U, Musch E, Mackes KG, Hartlapp JH (1985) Zytostatika-induzierte Lungenfibrose am Beispiel einer mit Melphalan behandelten Patientin mit Leichtkettenmyelom. Krebsmedizin 6: 25–28
Loos U, Musch E, Niese D, Baumm A (1986) Systemische Verfügbarkeit von Melphalan bei der intermittierenden Therapie des multiplen Myeloms. Krebsmedizin 7: 25–28
Osterheld HKO, Musch E, von Unruh GE, Loos U, Rauschecker H, Mühlenbruch BJ (1988) A sensitive high-performance liquid chromatographic assay for melphalan and its hydrolysis products in blood and plasma. Cancer Chemother Pharmacol 21: 156–162
Reece PA, Kotasek D, Morris RG, Dale BM, Sage RE (1986) The effect of food on oral melphalan absorption. Cancer Chemother Pharmacol 16: 194–197
Statistical Consultants, Inc. (1986) PCNONLIN and NON-LIN84: software for the statistical analysis of nonlinear models. Am Stat 40: 52
Sviland L, Robinson A, Proctor SJ, Bateman DN (1987) Interaction of cimetidine with oral melphalan. Cancer Chemother Pharmacol 20: 173–175
Taha IAK, Ahmad RAJ, Rogers HJ (1981) Melphalan estimation by quantitative thin-layer chromatography. Cancer Chemother Pharmacol 5: 181–184
Taha IAK, Ahmad RA, Gray H, Roberts CI, Rogers HJ (1982) Plasma melphalan and prednisolone concentrations during oral therapy for multiple myeloma. Cancer Chemother Pharmacol 9: 57–60
Taha IAK, Ahmad RA, Rogers DW, Pritchard J, Rogers HJ (1983) Pharmacokinetics of melphalan in children following high-dose intravenous injection. Cancer Chemother Pharmacol 10: 212–216
Tattersall MHN, Jarman M, Newlands ES, Holyhead L, Milstead RAV, Weinberg A (1978) Pharmaco-kinetics of melphalan following oral and intravenous administration in patients with malignant diesease. Europ J Cancer 14: 507–513
Vistica D (1983) Cellular pharmacokinetics of phenylalanine mustards. Pharmacol Ther 22: 379–422
Woodhouse KW, Hamilton P, Lennard A, Rawlins MD (1983) The pharmacokinetics of melphalan in patients with multiple myeloma: an intravenous/oral study using a conventional dose regimen. Eur J Clin Pharmacol 24: 283–285
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Loos, U., Musch, E., Engel, M. et al. The pharmacokinetics of melphalan during intermittent therapy of multiple myeloma. Eur J Clin Pharmacol 35, 187–193 (1988). https://doi.org/10.1007/BF00609251
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00609251