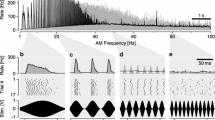
Summary
-
1.
Giant interneurons of the cockroachPeriplaneta americana show reduced responsiveness, for up to 800 ms, following activation by sound via their cereal inputs. The reduction in responsiveness is evident when tested either by a sound test stimulus, or by electrical stimulation of the cereal nerve.
-
2.
Afferent axons of cereal filiform hair sensilla exhibit a reduced responsiveness during and immediately following activation by sound.
-
3.
Use of oscillatory sound stimuli, rather than continuous deflections of the filiform hairs, circumvents the possibility that slow mechanical restitution of receptor elements after deflection is the cause of the post activity reduced responsiveness.
-
4.
The magnitude of reduction in responsiveness of cereal afferents closely parallels their post activity afterpotential.
-
5.
Amplitude and duration of the afterpotential were found to increase with stimulus intensity and to have the largest values at a stimulus frequency of 250 Hz and 300 ms duration.
-
6.
The afterpotential curve was found to fit the equationV t= V(1-e −t/τ) τ=60 ms, at stimulus frequency of 100 Hz.
-
7.
It is predicted that the reduced responsiveness of cereal afferents following activity will increase the threshold for escape immediately after a running bout.
Similar content being viewed by others
References
Adrian ED, Zotterman Y (1926) The impulses produced by sensory nerve-endings. part 2: The response of a single end organ. J Physiol (Lond) 61:151–171
Callec JJ (1974) Synaptic transmission in the central nervous system of insects. In: Trehene JE (ed) Insect neurobiology. American Elsevier, New York, pp 119–185
Camhi JM, Nolen TG (1981) Properties of the escape system of cockroaches during walking. J Comp Physiol 142:339–346
Dagan D, Camhi JM (1979) Responses to wind recorded from the cereal nerve of the cockroachPeriplaneta americana. II. Directional selectivity of the sensory neurones innervating single columns of filiform hairs. J Comp Physiol 133:103–110
Dagan D, Parnas I (1970) Giant fibre and small fibre pathways involved in the evasive response of the cockroach,Periplaneta americana. J Exp Biol 52:313–324
Dagan D, Volman S (1979) Behavioral and physiological localization of wind stimuli in first instar cockroaches. Neurosci Abstr 5:244
Dagan D, Volman S (1982) Sensory basis for directional wind detection in first instar cockroachesPeriplaneta americana. J Comp Physiol 147:471–478
Daley DL (1982) Neural basis of wind-receptive fields of cockroach giant interneurons. Brain Res 238 (in press)
Daley DL, Camhi JM (1981) Neural basis of the directional selectivity of wind-receptive fields of cockroach giant interneurons. Neurosci Abstr 7:252
Daley DL, Delcomyn F (1980) Modulation of the excitability of cockroach giant interneurones during walking. II. Central and peripheral components. J Comp Physiol 138:241–251
Eyzaguirre D, Kuffler SW (1955) Processes of excitation in the dendrites and in the soma of single isolated sensory nerve cells of the lobster and crayfish. J Gen Physiol 39:87–119
Florey E (1956) Adaptationserscheinungen in den sensiblen Neuronen des Streck-Receptoren des Flusskrebses. Z Naturforsch [B] 11:504–513
Gnatzy W (1976) The ultrastructure of the thread-hairs on the cerci of the cockroachPeriplaneta americana L.: The intermoult phase. J Ultrastruct Res 54:124–134
Katz B (1950) Depolarization of sensory terminals and the initiation of impulses in the muscle spindle. J Physiol (Lond) 111:261–282
Loewenstein WR, Mendelson M (1965) Components of receptor adaptation in a Pacinian corpuscle. J Physiol (Lond) 177:377–397
Margolin Y (1979) Cereal nerve regeneration in crickets: Synaptic inputs to ipsi- and contralateral giant interneurons. M Sc thesis, Technion Israel Institute of Technology, Haifa (in Hebrew)
Matthews BHC (1933) Nerve endings in mammalian muscle. J Physiol (Lond) 78:1–53
Murphey RK, Palka J (1974) Efferent control of cricket giant fibers. Nature 248:249–251
Nakajima S, Onodera K (1969) Adaptation of the generator potential in the crayfish stretch receptors under constant length and constant tension. J Physiol (Lond) 200:187–204
Nakajima S, Takahashi K (1966) Post-tetanic hyperpolarization and electrogenic Na pump in stretch receptor neurone of crayfish. J Physiol (Lond) 187:105–127
Nicklaus R (1965) Die Erregung einzelner Fadenhaare vonPeriplaneta americana in Abhängigkeit von der Grösse und Richtung der Auslenkung. Z Vergl Physiol 50:331–362
Orida N, Josephson RK (1978) Peripheral control of responsiveness to auditory stimuli in giant fibres of crickets and cockroaches. J Exp Biol 72:153–164
Ottoson D, Shepherd GM (1971) Transducer properties and integrative mechanisms in the frog's muscle spindle. In: Loewenstein WR (ed) Principles of receptor physiology. Springer, Berlin Heidelberg New York (Handbook of sensory physiology, vol I, pp 442–499)
Parnas I, Dagan D (1971) New aspects in insect giant axon function. Adv Insect Physiol 8:95–145
Plummer MR, Camhi JM (1981) Discrimination of sensory signals from noise in the escape system of the cockroach: The role of wind acceleration. J Comp Physiol 142:347–357
Schwartzkopff J (1974) Mechanoreception. In: Rockstein M (ed) The physiology of insecta, vol II. Academic Press, New York London, pp 273–352
Thurm U (1964) Das Receptorpotential einzelner mechanoreceptorischer Zellen von Bienen. Z Vergl Physiol 48:131–156
Thurm U (1965) An insect mechanoreceptor. II. Receptor potentials. Cold Spring Harbor Symp Quant Biol 30:83–94
Watanabe T, Shimada Z (1971) Auditory temporal masking: An electrophysiological study of single neurons in the cat cochlear nucleus and inferior colliculus. Jpn J Physiol 21:537–550
Westin J (1979) Responses to wind recorded from the cereal nerve of the cockroachPeriplaneta americana. I. Response properties of single sensory neurons. J Comp Physiol 133:97–102
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dagan, D., Pratt, H. & Margolin, Y. Post activity effects in mechanoreceptor afferents of the cockroach. J. Comp. Physiol. 150, 121–127 (1983). https://doi.org/10.1007/BF00605295
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00605295