Summary
-
1.
Intracellular recordings have been made from neuropilar processes of ventilatory motoneurones in the semi-isolated or isolated thoracic ganglion ofCarcinus maenas (Fig. 1). Cobalt backfills show that the two antagonistic sets of motor axons (Fig. 2) innervating the scaphognathite (SG) depressor and levator muscles, respectively, originate from a single dense neuropile within the ipsilateral hemiganglion (Fig. 3). Soma diameters range from 20 to 70 μm.
-
2.
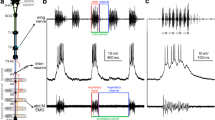
Axon spikes appear in the neurites of motoneurones as decremented, non-overshooting potentials (usually 5–15 mV in amplitude), suggesting that they arise distally at sites located near the point at which the axons leave the ganglion. Injection of small depolarizing currents (1–2 nA) elicited repetitive firing in all motoneurones penetrated, the firing frequency increasing monotonically with the current intensity (Figs. 4, 5). Conversely, hyperpolarizing current decreased or prevented any tonic spiking activity, and most cells showed rebound excitation on release from negative current. Rebound from hyperpolarization is probably the only mechanism intrinsic to the individual motoneurones which contributes to the generation of motoneurone bursts during normal rhythmic output.
-
3.
Large (8–30 mV) oscillations in membrane potential underlie spontaneous rhythmic bursting in ventilatory motoneurones (Figs. 6–9). These oscillations result mainly from cyclical inhibition between the impulse bursts at chemical synapses, since the waveform of both depressorand levator motoneurones could be reversed in polarity by maintained hyperpolarization with injected current. No evidence was found for phasic excitatory synaptic inputs to the ventilatory motoneurones, nor were any discrete (brief) unitary postsynaptic potentials observed in motoneuronal recordings.
-
4.
There is evidence for central inhibitory coupling between functionally antagonistic motoneurones, and for excitatory feedback from motoneurones to the premotor elements that drive them (Fig. 10). This indicates that the motoneurones themselves are components of the ventilatory central pattern generator.
-
5.
The application of 10−6mol/l tetrodotoxin (TTX) was used to suppress all impulse production in rhythmically active preparations (Figs. 11, 12). Under these conditions the continuation of synaptically-driven slow wave activity in the ventilatory motoneurones suggests that graded chemical synaptic interactions play an important role in the generation of the ventilatory motor programme. This in turn is consistent with earlier findings that the central drive to ventilatory motoneurones is derived from cyclic oscillations in membrane potential of non-spiking interneurones (Mendelson 1971; Simmers and Bush 1980).
Similar content being viewed by others
Abbreviations
- CNS :
-
central nervous system
- D(N) :
-
depressor (nerve)
- L(N) :
-
levator (nerve)
- (i.)p.s.p. :
-
(inhibitory) postsynaptic potential
- SG :
-
scaphognathite
- TTX :
-
tetrodotoxin
References
Anderson WW, Barker DL (1981) Synaptic mechanisms that generate network oscillations in the absence of discrete postsynaptic potentials. J Exp Zool 216:187–191
Arudpragasam KD, Naylor E (1964) Gill ventilation and the role of reversed respiratory currents inCarcinus maenas (L.). J Exp Biol 41:299–307
Bentley DR (1969) Intracellular activity in cricket neurons during generation of song patterns. Z Vergl Physiol 62:267–283
Berlind A (1977) Neurohumoral and reflex control of scaphognathite beating in the crabCarcinus maenas. J Comp Physiol 116:77–90
Burrows M (1975) Co-ordinating interneurones of the locust which convey two patterns of motor commands: their connexions with flight motoneurones. J Exp Biol 63:713–733
Burrows M (1982) Interneurones co-ordinating the ventilatory movements of the thoracic spiracles in the locust. J Exp Biol 97:385–400
Burrows M, Siegler MVS (1978) Graded synaptic transmission between local interneurones and motor neurones in the metathoracic ganglion of the locust. J Physiol (Lond) 285:231–255
Cannone AJ, Bush BMH (1980) Reflexes mediated by non-impulsive afferent neurones of thoracic coxal muscle receptor organs in the crab,Carcinus maenas. I. Receptor potentials and promotor motoneurone responses. J Exp Biol 86:257–303
Cannone AJ, Bush BMH (1982) Dual reflex motor control of non-spiking crab muscle receptor. I. Positive feedback tonically reduced and dynamically stabilized by concurrent inhibition of Rml. J Comp Physiol 148:365–377
Clark JV (1979) The neuronal basis of rhythmic pumping inBalanus hameri. J Comp Physiol 130:183–190
Delcomyn F (1980) Neural basis of rhythmic behaviour in animals. Science 210:492–498
Gillette R, Kovac MP, Davis WJ (1978) Command neurons inPleurobranchaea receive synaptic feedback from the motor network they excite. Science 199:798–801
Graubard K, Raper JA, Hartline DK (1980) Graded synaptic transmission between spiking neurons. Proc Natl Acad Sci USA 77:3733–3735
Grillner S (1981) Control of locomotion in bipeds, tetrapods and fish. In: Brooks VB (ed) Handbook of physiology, vol II, sect 1, part 2. American Physiological Society, Bethesda (Maryland), pp 1179–1236
Heitler WJ (1978) Coupled motoneurones are part of the crayfish swimmeret central oscillator. Nature 275:231–234
Heitler WJ, Pearson KJ (1980) Non-spiking interactions and local interneurones in the central pattern generator of the crayfish swimmeret system. Brain Res 187:206–211
Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol (Lond) 117:500–544
Hughes GM, Knights B, Scammell CA (1969) The distribution of PO2 and hydrostatic pressure changes within the branchial chambers in relation to gill ventilation of the shore crabCarcinus maenas L. J Exp Biol 51:203–220
Kennedy D, Davis WJ (1977) Organization of invertebrate motor systems. In: Geiger SR, Kandel ER, Brookhart JM, Mountcastle VB (eds) Handbook of physiology, vol I, sect 1, part 2. American Physiological Society (Bethesda) Maryland, pp 1023–1087
Kennedy D, Selverston AI, Remler MP (1969) Analysis of restricted neural networks. Science 164:1488–1496
Komatsu A (1980) Synaptic input driving respiratory motoneurons in dragonfly larvae. Brain Res 201:215–219
Maynard DM, Walton KD (1975) Effects of maintained depolarization of presynaptic neurons on inhibitory transmission in lobster neuropil. J Comp Physiol 97:215–243
Mendelson M (1971) Oscillator neurons in crustacean ganglia. Science 171:1170–1173
Moffett S (1977) Neuronal events underlying rhythmic behaviors in invertebrates. Comp Biochem Physiol 57:187–195
Moody-Corbett F, Pasztor VM (1980) Innervation, synaptic physiology and ultrastructure of three muscles of the second maxilla in crayfish. J Neurobiol 11:21–30
Narahashi T (1974) Chemicals as tools in the study of excitable membranes. Physiol Rev 54:813–889
Pasztor VM (1968) The neurophysiology of respiration in decapod Crustacea. I. The motor system. Can J Zool 46:585–596
Pasztor VM (1969) The neurophysiology of respiration in decapod Crustacea. II. The sensory system. Can J Zool 47:435–441
Pasztor VM, Bush BMH (1982) Impulse-coded and analog signaling in single mechanoreceptor neurons. Science 215:1635–1637
Pearson J (1908) Cancer. LMBC Mem Type Br Mar Pl Anim 16 pp, viii and 209. Williams and Margate, London
Pearson KG (1980) Burst generation in coordinating interneurons of the ventilatory system of the locust. J Comp Physiol 137:305–313
Pearson KG, Fourtner CR (1975) Nonspiking interneurons in walking system of the cockroach. J Neurophysiol 38:33–52
Perkel DH, Mulloney B (1974) Motor pattern production in reciprocally inhibitory neurons exhibiting postinhibitory rebound. Science 185:181–183
Pilkington JB, MacFarlane DW (1978) Numbers and central projections of crab second maxilla motoneurones. J Mar Biol Assoc UK 58:571–584
Pilkington JB, Simmers AJ (1973) An analysis of bailer movements responsible for gill ventilation in the crab,Cancer novae-zelandiae. Mar Behav Physiol 2:73–95
Pitman RM, Tweedle CD, Cohen MJ (1973) The form of nerve cells: determination by cobalt impregnation. In: Kater SB, Nicholson C (eds) Intracellular staining in neurobiology. Springer, Berlin Heidelberg New York, pp 83–97
Raper JA (1979) Non-impulse mediated synaptic transmission during the generation of a cyclic motor program. Science 205:304–306
Robertson RM, Pearson KG (1982) A preparation for the intracellular analysis of neuronal activity during flight in the locust. J Comp Physiol 146:311–320
Russell DF, Hartline DK (1978) Bursting neural networks: A reexamination. Science 200:453–456
Selverston AI, Russell DF, Miller JP, King DG (1976) The stomatogastric nervous system: structure and function of a small neural network. Prog Neurobiol 7:215–289
Siegler MVS (1981) Postural changes alter synaptic interactions between nonspiking interneurons and motor neurons of the locust. J Neurophysiol 46:310–323
Siegler MVS, Mpitsos GJ, Davis WJ (1974) Motor organization and generation of rhythmic feeding output in buccal ganglion ofPleurobranchaea. J Neurophysiol 37:1173–1196
Simmers AJ (1978) A preparation for analysis of the neural control of ventilation in crabs. J Physiol (Lond) 277:15–16P
Simmers AJ (1979) Oscillatory potentials in crab ventilatory neurones. J Physiol (Lond) 287:34–40P
Simmers AJ (1981) Non-spiking interactions in crustacean rhythmic motor systems. In: Roberts A, Bush BMH (eds) Neurones without impulses. Cambridge University Press, Cambridge, pp 177–198
Simmers AJ (1981) Central neuronal mechanisms underlying rhythmic ventilatory behaviour in the shore crabCarcinus maenas. Ph D thesis, University of Bristol
Simmers AJ, Bush BMH (1980) Non-spiking neurones controlling ventilation in crabs. Brain Res 197:247–252
Simmons PJ (1977) Neuronal generation of singing in a cicada. Nature 270:243–245
Tazaki K, Cooke IM (1979) Isolation and characterization of slow, depolarizing responses of cardiac ganglion neurons in the crab,Portunus sanguinolentus. J Neurophysiol 42:1000–1021
Wilkens JL, Young RE (1975) Patterns and bilateral coordination of scaphognathite rhythms in the lobsterHomarus americanus. J Exp Biol 63:219–235
Wyman RJ (1977) Neural generation of the breathing rhythm. Ann Rev Physiol 39:417–448
Wyse GA (1972) Intracellular and extracellular motor neuron activity underlying rhythmic respiration inLimulus. J Comp Physiol 81:259–276
Young RE (1975) Neuromuscular control of ventilation in the crab,Carcinus maenas. J Comp Physiol 101:1–37
Young RE, Coyer PE (1979) Phase coordination in the cardiac and ventilatory rhythms of the lobsterHomarus americanus. J Exp Biol 82:53–74
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Simmers, A.J., Bush, B.M.H. Central nervous mechanisms controlling rhythmic burst generation in the ventilatory motoneurones ofCarcinus maenas . J. Comp. Physiol. 150, 1–21 (1983). https://doi.org/10.1007/BF00605283
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00605283