Summary
-
1.
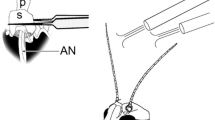
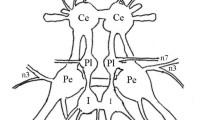
This paper describes the operation of synapses which large, second-order neurones of each ocellus of a locust (L-neurones) make with a pair of large, identified descending neurones on either side of the brain (Fig. 1). Intracellular recordings were made from the axons of the L-neurones, and usually from the cell bodies of the third-order neurones.
-
2.
The third-order neurones respond, in addition to changes in illumination, to currents of air which deflect wind sensitive hairs on the head (Fig. 2B).
-
3.
Both L-neurones and the third-order neurones hyperpolarise when their ocellus is illuminated (Figs. 2, 3). The hyperpolarising responses of the third-order neurones saturate at a light intensity which is well within the range of intensities to which the L-neurones respond (Fig. 3). L-neurones and third-order ocellar neurones produce sharply rising regenerative responses when a bright light is switched off, and the third-order neurones spike at less intense changes in illumination than L-neurones do (Fig. 3A).
-
4.
L-neurones make excitatory chemical synapses with the third-order ocellar neurones (Figs. 4, 5). In steady daylight illumination, L-neurones continually release transmitter onto the third-order neurones (Fig. 4). The hyperpolarising responses of the third-order neurones to increases in illumination are due to decreases in the rate of release of transmitter from the L-neurones.
Similar content being viewed by others
References
Autrum H, Zettler F, Järvilehto M (1970) Postsynaptic potentials from a single monopolar neurone of the ganglion opticum 1 of the blowflyCalliphora. Z Vergl Physiol 70:414–424
Bacon J, Altman JS (1977) A silver intensification method for cobalt-filled neurones in wholemount preparations. Brain Res 138:359–363
Baylor DA, Fettiplace R (1977) Transmission from photoreceptors to ganglion cells in turtle retina. J Physiol 271:391–429
Blight AR, Llinás R (1980) The non-impulsive stretch receptor complex of the crab: a study of depolarisation — release coupling at a tonic sensorimotor synapse. Philos Trans R Soc Lond [Biol] 290:219–276
Burrows M, Siegler MVS (1978) Graded synaptic transmission between local interneurones and motoneurones in the metathoracic ganglion of the locust. J Physiol 285:231–255
Chappell RL, Dowling JE (1972) Neural organization of the median ocellus of the dragonfly. 1. Intracellular electrical activity. J Gen Physiol 60:121–147
Dowling JE, Ripps JH (1973) Effects of magnesium on horizontal cell activity in skate retina. Nature 242:101–103
Eibl E (1978) Morphology of the sense organs in the proximal parts of the tibiae ofGryllus campestris andGryllus bimaculatus de Geer (Insecta, Ensifera). Zoomorphologie 89:185–205
Goodman CS (1976) Anatomy of the ocellar interneurones of acridid grasshoppers. 1. The large interneurones. Cell Tissue Res 175:183–202
Goodman CS, Williams JLD (1976) Anatomy of the ocellar interneurones of acridid grasshoppers. II. The small interneurones. Cell Tissue Res 175:203–225
Goodman LJ, Patterson JA, Mobbs PG (1975) The projection of ocellar nerves within the brain of the locustSchistocerca gregaria. Cell Tissue Res 157:467–492
Goodman LJ, Mobbs PG, Guy RG (1977) Information processing along the course of a visual interneuron. Experientia 33:748–750
Graubard K (1978) Synaptic transmission without action potentials. Input-output properties of a nonspiking presynaptic neurone. J Neurophysiol 41:1014–1025
Guy RG, Goodman LJ, Mobbs PG (1977) Ocellar connections with the ventral nerve cord of the locustSchistocerca gregaria: electrical and anatomical characteristics. J Comp Physiol 115:337–350
Katz B, Miledi R (1967) A study of synaptic transmission in the absence of nerve impulses. J Physiol 192:409–426
Laughlin SB (1973) Neural integration in the first optic neuropile of dragonflies. I. Signal amplification in dark-adapted second-order neurones. J Comp Physiol 84:333–355
Laughlin SB (1981) Neural principles in the visual system. In: Autrum H (ed) Comparative Physiology and evolution of vision in invertebrates B. Springer, Berlin Heidelberg New York. (Handbook of sensory physiology, vol VII/6B, pp 135–280)
Laughlin SB, Hardie RC (1978) Common strategies for light adaptation in the peripheral visual systems of fly and dragonfly. J Comp Physiol 128:319–340
Martin AR, Ringham GL (1975) Synaptic transfer at a vertebrate central nervous synapse. J Physiol 251:409–426
Miller RF (1979) The neuronal basis of ganglion-cell receptive field organisation and the physiology of amacrine cells. In: Schmitt FO, Worden FG (eds) The neurosciences, fourth study Programm. M.I.T. Press, Cambridge, MA
Normann RA, Werblin FS (1974) Control of retinal sensitivity. I. Light and dark-adaptation of vertebrate rods and cones. J Gen Physiol 63:37–61
Oertel DA, Stuart AE (1981) Transformation of signals by interneurones in the barnacle's visual pathway. J Physiol 311:127–146
O'Shea M, Rowell CHF, Williams JLD (1974) The anatomy of a locust visual interneurone; the descending contralateral movement detector. J Exp Biol 60:1–12
Parry DA (1947) The function of the insect ocellus. J Exp Biol 24:211–219
Patterson JA, Goodman LJ (1974) Intracellular responses of receptor cells and second order cells of the ocelli of the locustSchistocerca gregaria. J Comp Physiol 95:237–250
Shaw SR (1968) Organisation of the locust retina. Symp Zool Soc (Lond) 23:135–163
Simmons PJ (1980) A locust wind and ocellar brain neurone. J Exp Biol 85:281–294
Simmons PJ (1981) Ocellar excitation of the DCMD: an identified locust interneurone. J Exp Biol 91:355–359
Strausfeld NJ, Obermayer M (1976) Resolution of intraneuronal and transynaptic migration of cobalt in the insect visual and nervous systems. J Comp Physiol 110:1–12
Svaetichin A, MacNichol EF (1958) Retinal mechanisms for chromatic and achromatic vision. Ann NY Acad Sci 72:385–404
Tyrer NM, Bacon JP, Davies CA (1979) Sensory projections from wind-sensitive head hairs of the locustSchistocerca gregaria. Distribution in the central nervous system. Cell Tissue Res 203:79–92
Werblin FS (1974) Control of retinal sensitivity. II. Lateral interactions at the outer plexiform layer. J Gen Physiol 63:62–87
Williams JLD (1975) Anatomical studies of the insect central nervous system: a groundplan of the midbrain and on introduction to the central complex in the locustSchistocerca gregaria. J Zool (Lond) 176:67–86
Wilson M (1978a) The functional organisation of locust ocelli. J Comp Physiol 124:297–316
Wilson M (1978b) Generation of graded potential signals in the second order cells of locust ocellus. J Comp Physiol 124:317–331
Wilson M (1978c) The origin and properties of discrete hyperpolarising potentials in the second order cells of locust ocellus. J Comp Physiol 128:347–358
Author information
Authors and Affiliations
Additional information
I am grateful to Malcolm Burrows for his advice and encouragement.
Rights and permissions
About this article
Cite this article
Simmons, P.J. Synaptic transmission between second- and third-order neurones of a locust ocellus. J. Comp. Physiol. 145, 265–276 (1981). https://doi.org/10.1007/BF00605039
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00605039