Summary
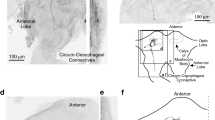
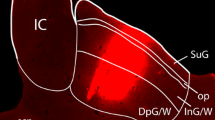
The minor branch of the tritocerebral commissure of the locust,Locusta migratoria, contains only two axons which are from interneurons in the brain descending to the ventral cord ganglia. The smaller of these two neurons, the tritocerebral commissure dwarf (TCD), is immunoreactive to GABA, suggesting that it may be an inhibitory interneuron. We have exploited the accessibility of its axon in the commissure, first, to fill it with cobalt to define its morphology, and second, to record its input characteristics. It has a cell body and arborization of fine branches in the deutocerebrum of the brain, its axon passes contralateral through the tritocerebral commissure and it forms bilateral arborizations in the suboesophageal and three thoracic ganglia. It receives mechanosensory input from many regions of the ipsilateral body and head, and it is sensitive to illumination levels, generally showing greater spontaneous activity in the dark.
It is one of the largest GABA-immunoreactive descending interneurons in the locust, suggesting it plays a prominent role in behaviour. Since it is easily accessible for physiological recording, its roles in circuits for particular components of behaviour should be amenable to investigation.
Similar content being viewed by others
References
Altman JS, Kien J (1987) A model for decision making in the insect nervous system. In: Ali MA (ed) Nervous systems in invertebrates. Plenum, NY, pp 621–643
Ammermüller J, Weiler R (1985) S-neurons and not L-neurons are the source of GABAergic action on the ocellar retina. J Comp Physiol A 157:779–788
Bacon JP, Altman JS (1977) A silver intensification method for cobalt-filled neurons in wholemount preparations. Brain Res 138:359–363
Bacon JP, Möhl B (1979) Activity of an identified wind interneurone in a flying locust. Nature 278:638–640
Bacon JP, Möhl B (1983) The tritocerebral commissure giant (TCG) wind-sensitive interneurone in the locust. I. Its activity in straight flight. J Comp Physiol 150:439–452
Bacon JP, Tyrer NM (1978) The tritocerebral commissure giant (TCG): a bimodal interneurone in the locust,Schistocerca gregaria. J Comp Physiol 126:317–325
Bicker G, Schäfer S, Kingan TG (1985) Mushroom body feedback interneurones in the honeybee show GABA-like immunoreactivity. Brain Res 360:394–397
Bräunig P, Pflüger HJ, Hustert R (1983) The specificity of central nervous projections of locust mechanoreceptors. J Comp Neurol 218:197–207
Burrows M (1980) The control of sets of motoneurons by local interneurones in the locust. J Physiol (Lond) 298:213–233
Burrows M (1987) Parallel processing of proprioceptive signals by local interneurones and motor neurones in the locust. J Neurosci 7:1064–1080
Burrows M, Pflüger HJ (1986) Processing by local interneurones of mechanosensory signals involved in a leg reflex of the locust. J Neurosci 6:2764–2777
Burrows M, Siegler MVS (1976) Transmission without spikes between locust interneurones and motor neurones. Nature 262:222–224
Burrows M, Siegler MVS (1982) Spiking local interneurones mediate local reflexes. Science 217:659–662
Burrows M, Siegler MVS (1984) The morphological diversity and receptive fields of spiking local interneurons in the locust metathoracic ganglion. J Comp Neurol 224:483–508
Burrows M, Watkins BL (1986) Spiking local interneurones in the mesothoracic ganglion of the locust: homologies with metathoracic interneurones. J Comp Neurol 245:29–40
Emson PC, Burrows M, Fonnum F (1974) Levels of glutamate decarboxylase, choline acetyltransferase and acetyl choline esterase in identified motor neurones of the locust. J Neurobiol 5:33–42
Griss C, Rowell CHF (1986) Three descending interneurons reporting deviation from course in the locust. 1. Anatomy. J Comp Physiol A 158:765–774
Hale JP, Burrows M (1985) Innervation patterns of inhibitory motor neurones of the locust. J Exp Biol 117:401–413
Hedwig B (1986a) On the role in stridulation of plurisegmental interneurons of the acridid grasshopperOmocestus viridulus L. 1. Anatomy and physiology of descending cephalothoracic interneurons. J Comp Physiol A 158:413–427
Hedwig B (1986b) On the role in stridulation of plurisegmental interneurons of the acridid grasshopperOmocestus viridulus L. II. Anatomy and physiology of ascending and T shaped interneurons. J Comp Physiol A 158:429–444
Hensler K (1988) Intersegmental interneurons involved in the control of head movements in crickets. J Comp Physiol 162:111–126
Horsmann U (1985) Der Einfluß proprioceptiver Windmessung auf den Flug der Wanderheuschrecke und die Bedeutung descendierender Neuronen der Tritocerebralkommissur. PhD thesis, Universität Köln, W. Germany
Hoskins SG, Homberg U, Kingan T, Christensen TA, Hildebrand JG (1986) Immunocytochemistry of GABA in the antennal lobes of the sphinx mothManduca sexta. Cell Tissue Res 244:243–252
Kien J (1983) The initiation and maintenance of walking in the locust. An alternative to the command concept. Proc R Soc Lond B 219:137–174
Kien J, Altman JS (1984) Descending interneurones from the brain and suboesophageal ganglia and their role in the control of locust behaviour. J Insect Physiol 30:59–72
Kravitz EA, Kuffler SW, Potter DD (1963) Gamma-aminobutyric acid and other blocking compounds in the Crustacea. III. Their relative concentrations in separated motor and inhibitory axons. J Neurophysiol 26:739–751
Laurent G (1986) Thoracic intersegmental interneurones in the locust with mechanosensory inputs from a leg. J Comp Physiol A 159:171–186
Laurent G (1987a) The morphology of a population of thoracic intersegmental interneurones in the locust. J Comp Neurol 256:412–429
Laurent G (1987b) Parallel effects of joint receptors on motor neurones and intersegmental interneurones in the locust. J Comp Physiol A 160:341–353
Laurent G (1988) Local circuits underlying excitation and inhibition of intersegmental interneurones in the locust. J Comp Physiol A 162:145–157
Laurent G, Burrows M (1988) Direct excitation of non-spiking local interneurones by exteroreceptors underlies tactile reflexes in the locust. J Comp Physiol A 162:563–572
Meyer EP, Matute C, Streit P, Nässle DR (1986) Insect optic lobe neurons identifiable with monoclonal antibodies to GABA. Histochemistry 84:207–216
Möhl B, Bacon JP (1983) The tritocerebral commissure giant (TCG) wind sensitive interneurone in the locust. II. Directional sensitivity and role in flight stabilization. J Comp Physiol 150:453–465
Nässle DR (1987) Neuroactive substances in the insect CNS. In: Ali MA (ed) Nervous systems in invertebrates. Plenum, NY, pp 171–212
Otsuka M, Obata K, Miyata Y, Tanaka Y (1971) Measurement of aminobutyric acid in isolated nerve cells of cat central nervous system. J Neurochem 18:287–295
Pearson KG, Robertson RM (1981) Interneurons co-activating hindleg flexor and extensor motoneurons in the locust. J Comp Physiol 144:391–400
Robertson RM, Pearson KG (1983) Interneurons in the flight system of the locust: distribution, connections, and resetting properties. J Comp Neurol 215:33–50
Römer H, Marquart V (1984) Morphology and physiology of auditory interneurons in the metathoracic ganglion of the locust. J Comp Physiol A 155:249–262
Rowell CHF, Reichert H (1986) Three descending interneurons reporting deviation from course in the locust. II. Physiology. J Comp Physiol A 158:775–794
Schäfer S, Bicker G (1986) Distribution of GABA-like immunoreactivity in the brain of the honeybee. J Comp Neurol 246:287–300
Shepherd D (1984) Some aspects of inhibition in the peripheral and central nervous systems of the locustSchistocerca gregaria. PhD thesis, University of Manchester, UK
Siegler MVS, Burrows M (1984) The morphology of two groups of spiking local interneurons in the metathoracic ganglion of the locust. J Comp Neurol 224:463–482
Siegler MVS, Burrows M (1986) Receptive fields of motor neurons underlying local tactile reflexes in the locust. J Neurosci 6:507–513
Tyrer NM, Altman JS (1974) Motor and sensory flight neurones in a locust demonstrated using cobalt chloride. J Comp Neurol 157:117–138
Tyrer NM, Gregory GE (1982) A guide to the neuroanatomy of locust suboesophageal and thoracic ganglia. Phil Trans R Soc Lond Ser B 297:91–123
Tyrer NM, Bacon J, Davies CA (1979) Sensory projections from wind-sensitive head hairs of the locustSchistocerca gregaria. Cell Tissue Res 203:79–92
Usherwood PNR, Cull-Candy SG (1975) Pharmacology of somatic nerve-muscle synapses. In: Usherwood PNR (ed) Insect muscle. Academic Press, London, pp 207–280
Usherwood PNR, Grundfest H (1965) Peripheral inhibition in skeletal muscle of insects. J Neurophysiol 28:497–518
Watkins BL, Burrows M, Siegler MVS (1985) The structure of locust nonspiking interneurones in relation to the anatomy of their segmental ganglion. J Comp Neurol 240:233–255
Watson AHD (1986) The distribution of GABA-like immunoreactivity in the thoracic nervous system of the locustSchistocerca gregaria. Cell Tissue Res 246:331–341
Watson AHD, Burrows M (1985) The distribution of synapses on the two fields of neurites of spiking local interneurones in the locust. J Comp Neurol 240:219–232
Watson AHD, Burrows M (1987) Immunocytochemical and pharmacological evidence for GABAergic spiking local interneurons in the locust. J Neurosci 7:1741–1751
Watson AHD, Pflüger HJ (1987) The distribution of GABA-like immunoreactivity in relation to ganglion structure in the abdominal nerve cord of the locust. Cell Tissue Res 249:391–402
Watson AHD, Burrows M, Hale JP (1985) The morphology and ultrastructure of common inhibitory motor neurones in the thorax of the locust. J Comp Neurol 239:341–359
Weis-Fogh T (1949) An aerodynamic sense organ stimulating and regulating flight in locusts. Nature 164:873–874
Wohlers D, Huber F (1978) Intracellular recording and staining of cricket auditory interneurones inGryllus campestris andGryllus bimaculatus (de Geer). J Comp Physiol 127:11–28
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tyrer, N.M., Pozza, M.F., Humbel, U. et al. The tritocerebral commissure ‘dwarf’ (TCD): a major GABA-immunoreactive descending interneuron in the locust. J. Comp. Physiol. 164, 141–150 (1988). https://doi.org/10.1007/BF00603946
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00603946