Abstract
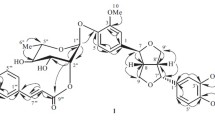
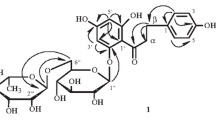
The structure of terpenoid and carbohydrate moieties of the molecule of reoselin have been confirmed by the use of13C NMR spectra, and the position of the carbohydrate moiety has been corrected. Reoselin is S-(−)-karatavikinol β-sophoroside at the tertiary hydroxy group (S-(−)-karativikinol tertiary-O-β-D-sophoroside) and not at the secondary hydroxy group, as was previously assumed. The absolute configuration of the single asymmetric center, the carbon atom to which the secondary hydroxy group is attached, has been assigned by analogy with S-(−)-epoxysqualene and S-(−)-epoxyfarnesol. S-(−)-Karatavikinol tertiary O-β-D-sophoroside has been isolated from giant fennels, a characteristic feature of which is the biosynthesis via direct antiparallel cyclization of bicyclic sesquiterpenoid derivatives of umbelliferone with the configuration of the ring linkage enantiometric to the linkage of rings A and B of triterpenoids.
Similar content being viewed by others
Literature cited
M. P. Kir'yalov and S. D. Movchan, Dokl. Akad. Nauk SSSR,148, No. 5, 1081 (1963).
N. P. Kir'yalov, T. V. Bukreeva, V. A. Gindin, G. M. Mamatov, and I. S. Kozhina, Khim. Prir. Soedin., 87 (1975).
M. P. Kir'yalov and V. Yu. Bagirov, Khim. Prir. Soedin., 225 (1969).
M. P. Kir'yalov and V. Yu. Bagirov, Khim. Prir. Soedin., 223 (1967).
Yu. Bagirov, Author's abstract of dissertation for the degree of Candidate of Chemical Sciences, Leningrad, 1973.
A. Sh. Kadyrov, A. I. Saidkhodzhaev, and G. K. Nikonov, Khim. Prir. Soedin., 574 (1975).
Ching-ejr Chang, H. G. Floss, and W. Steck, J. Org. Chem.,42, No. 8, 1337 (1977).
D. E. Dorman, M. Jautelat, and J. D. Roberts, J. Org. Chem.,36, No. 19, 2757 (1971).
C. Nishino and W. S. Bowers, Tetrahedron,32, 2875 (1976).
J. M. Browning and D. R. Martens, Tetrahedron,33, 931 (1977).
K. R. Markham, B. Ternai, R. Stanley, G. Geiger, and T. Mabry, Tetrahedron,34, 1389 (1978).
S. Seo, Y. Tomito, K. Tori, and Y. Yoshimura, J. Am. Chem. Soc.,100, No. 11, 3331 (1978).
T. Shishiboru, T. Fukui, and T. Suga, Chem. Lett., 1137 (1973).
Y. Suzuko, and S. Marumo, Tetrahedron, 1887 (1972).
Y. Suzuki and S. Marumo, J. Chem. Soc. Chem. Commun., 1199 (1971).
Additional information
V. L. Komarov Botanical Institute, Academy of Sciences of the USSR, Leningrad. Translated from Khimiya Prirodnykh Soedinenii, No. 1, pp. 62–66, January–February, 1987.
Rights and permissions
About this article
Cite this article
Bukreeva, T.V. Structure of the coumarin glycoside reoselin from the roots ofFerula kirialovii . Chem Nat Compd 23, 51–54 (1987). https://doi.org/10.1007/BF00602458
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00602458