Abstract
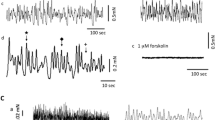
Electrical and mechanical properties and neuro-effector transmission were studied in circular strips of smooth muscle taken from the ileocecal junction of guinea-pigs in relation to sphincter action, using the microelectrode, and tension recording methods. The membrane potential of the smooth muscle was low (−43 mV) compared with the membrane potential of circular muscle cells of the ileum or caecum (−58 mV or −62mV). Only small populations of the muscle cells (about 5%) generated spontaneous action potentials.
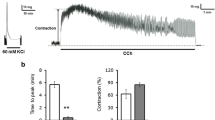
Field stimulation of the tissue produced an initial slight relaxation followed by a contraction, and the mechanical responses were accompanied by membrane hyperpolarization (i. j. p.) followed by repolarization with rebound spikes. Treatment with atropine increased the amplitude of i.j. ps and decreased the amplitue of the rebound repolarization. Propranolol or phentolamine did not affect the amplitude of i. j. p., however, phentolamine slightly reduced the amplitude of the rebound repolarization.
These results indicate that the ileocecal junction is predominantly controlled by non-adrenergic, non-cholinergic inhibitory nerve fibres and that the distribution of adrenergic and cholinergic excitatory nerve fibres is sparse.
Similar content being viewed by others
References
Abe Y, Tomita T (1968) Cable properties of smooth muscle. J Physiol 196:87–100
Bolton TB (1979) Mechanism of action of transmitters and other substances on smooth muscle. Physiol Rev 59:606–718
Bozler E (1948) Conduction, automaticity and tonus of visceral muscle. Experientia 4:213–218
Bülbring E, Den Hertog A (1980) The action of isoprenaline on the smooth muscle of the guinea-pig taenia-coli. J Physiol 304:277–296
Bülbring E, Kuriyama H (1963) Effect on changes in external sodium and calcium concentration of spontaneous electrical activity in smooth muscle of guinea-pig taenia coli. J Physiol 166:29–58
Bülbring E, Kuriyama H (1973) The action of catecholamines on guinea-pig taenia coli. Phil Trans R Soc Lond [Biol] 265:115–121
Bülbring E, Szurszewski J (1974) The stimulant action of noradrenaline (α-action) on guinea-pig myometrium compared with that of acetylcholine. Proc R Soc London [Biol] 185:225–262
Burnstock G, Brown CM (1981) An introduction to purinergic receptors. In: Burnstock G (ed) Purinergic receptors Chapman and Hall, London New York, p 1
Bywater RAR, Holman ME, Taylor GS (1980) Atropine-resistant depolarization in the guinea-pig small intestine. J Physiol 316:369–378
Chang PY, Hsu FY (1942) The localization of the intestinal inhibitory reflex arc. Quart J Exp Physiol 31:311–318
Cohen S, Harris L, Levitan R (1968) Manometric characteristics of the human ileocecal junctional zone. Gastroenterology 54:72–75
Creed KE (1975) Membrane properties of the smooth muscle cells of the rat anococcygeus muscle. J Physiol 245:49–62
Creed KE (1979) Functional diversity of smooth muscle. Br Med Bull 35:243–247
Elliott JR (1904) On the innervation of the ileo-colic sphincter. J Physiol 31:157–168
Gazet JC, Jarrett RJ (1964) The ileocaeco-colic sphincter studies in vitro in man, monkey, cat and dog. Br J Surg 51:368–370
Hinrichsen J, Ivy AC (1931) Studies of the ileo-cecal sphincter of the dog. Am J Physiol 96:494–507
Kuriyama H (1968) Ionic basis of smooth muscle action potentials. In: Charles FC (ed) Handbook of physiology, Alimentary Canal, Section 6. Cap 87. American Physiological Society, Washington DC, p 1767
Magaribuchi T, Ito Y, Kuriyama H (1971) Effects of catecholamines on the guinea-pig vas deference in various ionic environments. Jpn J Physiol 22:253–270
Pahlin PE, Kewenter J (1975) Reflexogenic contraction of the ileo-cecal sphincter in the cat following small or large intestinal distension. Acta Physiol Scand 95:126–132
Pahlin PE, Kewenter J (1976) The direct sympathetic nervous control of the cat ileo-cecal sphincter. Am J Physiol 231:296–305
Rubin MR, Fournet J, Snape WJ, Cohen S (1980) Adrenergic regulation of ileocecal sphincter function in the cat. Gastroenterology 78:15–21
Somlyo AP, Somlyo AV (1968) Vascular smooth muscle I. Normal structure, pathology, biochemistry, and biophysics. Pharmacol Rev 20:197–272
Suzuki H, Morita K, Kuriyama H (1976) Innervation and properties of the smooth muscle of the dog trachea. Jpn J Physiol 26:303–320
Taranenko BM, Shuba MF (1970) Mechanism of excitatory action of noradrenaline and adrenaline on portal vein smooth muscle cells. Neurophysiology USSR 2:643–653
Tomita T, Tokuno H, Usune S (1977) Confirmation of conductance increase by adrenaline in the guinea-pig taenia coli (α-action). Proc R Soc London [Biol] 198:473–478
Ulin AW, Deutch J (1950) Visualization of ileocecal papilla in a living subject. Gastroenterology 16:444–449
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kubota, M. Electrical and mechanical properties and neuro-effector transmission in the smooth muscle layer of the guinea-pig ileocecal junction. Pflugers Arch. 394, 355–361 (1982). https://doi.org/10.1007/BF00583701
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00583701