Abstract
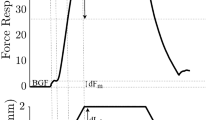
The study was designed to determine the influence of increased daily neuromuscular activity on the sprouting response of motoneurones following partial denervation. Female Sprague-Dawley rats had one hindlimb partially denervated by transecting lumbar radicular nerve L4, and were subsequently subjected to a daily programme of increased activity, including grid climbing and voluntary wheel exercise, for 9 days. Functional sprouting was estimated on day 10 by comparing the L5-evoked plantaris muscle forces, measured in situ, with those of the contralateral L5. Comparisons were made between responses from exercised and non-exercised rats. Tetanic (200 Hz) contribution of L5 axons to plantaris muscle force doubled during the period following partial denervation, but did not attain the equivalent of whole muscle tetanic tension of normal controls. Twitch: tetanic ratios were elevated, and tetanic contractions “fatigued” to a greater extent in partially denervated muscles, signifying limitations on the part of each sprouting motoneurone to tetanically activate its enlarged complement of muscle fibres. No influence of daily exercise following the lesion on any of these functional indices of motoneurone sprouting was evident. Increased daily neuromuscular activity, performed within the restrictions imposed by the neuromuscular deficit, does not influence motoneurone sprouting responses. This is in contrast to the enhancement of sprouting previously reported for motoneurones of rats subjected to intense, prolonged, daily exercise preceding the partial denervation, and when neurones remaining following partial denervation are electrically stimulated for relatively short periods of time (1 h), the day of the lesion.
Similar content being viewed by others
References
Brown MC, Holland RL, Hopkins WG (1981) Motor nerve sprouting. Annu Rev Neurosci 4:17–42
Brown MC, Ironton R (1978) Sprouting and regression of neuromuscular synapses in partially denervated mammalian muscles. J Physiol 278:325–348
Carmignoto G, Finesso M, Siliprandi R, Gorio A (1983) Muscle reinnervation. I. Restauration of transmitter release mechanisms. Neuroscience 8:393–401
Gardiner PF, Michel R, Iadeluca G (1984a) Previous exercise training influences functional sprouting of rat hindlimb motoneurons in response to partial denervation, Neurosci Lett 45:123–127
Gardiner PF, Michel R, Olha AE, Pettingrew F (1984b) Physiological properties of single motor units in rat plantaris following partial denervation. Abstr Soc Neurosci 10:782
Gerchman LB, Edgerton VR, Carrow RE (1975) Effects of physical training on the histochemistry and morphology of ventral motor neurons. Exp Neurol 49:790–801
Grafstein B, McQuarrie I (1978) Role of the nerve cell body in axonal regeneration. In: Cotman C (ed) Neuronal plasticity. Raven Press, New York, pp 155–195
Guth L, Smith S, Donati EJ (1980) Induction of intramuscular collateral nerve sprouting by neurally applied colchicine. Exp Neurol 67:513:523
Herbison GJ, Jaweed MM, Ditunno JF, Scott CM (1973) Effect of overwork during reinnervation of rat muscle. Exp Neurol 41:1–14
Hoffman H (1952) Acceleration and retardation of the process of axon-sprouting in partially denervated muscles. Aust J Exp Biol Med Sci 30:541–566
Holloszy JO, Booth FW (1976) Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol 38:273–291
Lewis DM, Luck JC, Knott SA (1972) A comparison of isometric contractions of the whole muscle with those of motor units in a fast-twitch muscle in the cat. Exp Neurol 37:68–85
Maehlen J, Nja A (1982) The effects of electrical stimulation on sprouting after partial denervation of guinea-pig sympathetic ganglion cells. J Physiol 322:151–166
Ribchester RR Taxt T (1984) Repression of inactive motor nerve terminals in partially denervated rat muscle after regeneration of active motor axons. J Physiol 347:497–511
Slack JR, Hopkins WG (1982) Neuromuscular transmission at terminals of sprouted mammalian motor neurones. Brain Res 237:121–135
Tal M, Rotshenker S (1983) Motor neuron sprouting following contralateral axotomy in mammalian muscles. Abstr Soc Neurosci 9:983
Westerman RA, Dennett X, Chan HS, Jedwab JD, Sriratana D, Ziccone SP (1979) Muscle hypertrophy after partial denervation: a feline model. In: Kidman AD, Tomkins JK (eds) Muscle, nerve and brain degeneration. Excerpta Medica, Amsterdam Oxford pp 139–150
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gardiner, P.F., Faltus, R.E. Contractile responses of rat plantaris muscles following partial denervation, and the influence of daily exercise. Pflugers Arch. 406, 51–56 (1986). https://doi.org/10.1007/BF00582952
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00582952