Abstract
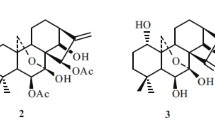
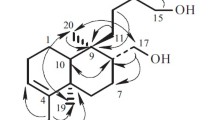
A new withasteroid has been isolated fromPhysalis viscosa L. — 28-hydroxywithaphysanolide, with mp 234°C, composition C28H38O8. The13C NMR spectra of a number of withasteroids have been investigated. For 28-hydroxywithaphysanolide we propose the structure 4β,14α,17β,20R,28-pentahydroxy-l-oxo-22R-witha-2,5,24-trienolide. The corresponding corrections have been made in the structural formulas of withaphysanolide and physalactone.
Similar content being viewed by others
Literature cited
R. N. Tursunova, V. A. Maslennikova, and N. K. Abubakirov, Khim. Prir. Soedin., 187 (1981).
V. A. Maslennikova, R. N. Tursunova, K. L. Seitanidi, and N. K. Abubakirov, Khim. Prir. Soedin., 214 (1980).
M. J. Begley, L. Crombie, P. J. Ham, and D. A. Whiting, J. Chem. Soc. Perkin Trans. I, 296 (1976).
A. K. Kalla, M. L. Raina, K. L. Dhar, M. A. Qurishi, and G. Snatze, Phytochemistry,18, 637 (1979).
S. W. Pelletier, N. V. Mody, J. Novacki, and J. Battacharyya, J. Nat. Prod.,42, 512 (1979).
H. E. Gottlieb and I. Kirson, Org. Magn. Reson.,16, 20 (1981).
S. W. Pelletier, G. Gebeyehu, J. Nowacki, and N. V. Mody, Heterocycles,15, 317 (1981).
R. B. Bates and D. J. Eckert, J. Am. Chem. Soc.,94, 8258 (1972).
R. B. Bates and S. R. Morehead, J. Chem. Soc. Chem. Commun., 125 (1974).
I. Kirson and H. E. Gottlieb, J. Chem. Research (S), 338 (M), 4257 (1980).
A. Abraham, I. Kirson, D. Lavie, and E. Glotter, Phytochemistry,14, 189 (1975).
V. A. Maslennikova, R. N. Tursunova, and N. K. Abubakirov, Khim. Prir. Soedin., 531 (1977).
I. Kirson and E. Glotter, J. Nat. Prod.,44, 633 (1981).
K. Sakurai, H. Ishii, S. Kobayashi, and T. I. Wao, Chem. Pharm. Bull.,24, 1403 (1976).
I. Kirson, A. Abraham, P. D. Sethi, S. S. Subramanian, and E. Glotter, Phytochemistry,15, 340 (1976).
R. N. Tursunova, V. A. Maslennikova, and N. K. Abubakirov, Khim. Prir. Soedin., 91 (1978).
S. M. Kupchan, R. W. Doskotch, P. Bollinger, A. T. McPhail, G. A. Sim, and J. A. SaenzRenauld, J. Am. Chem. Soc.,87, 5805 (1965).
S. M. Kupchan, W. K. Anderson, P. Bollinger, R. W. Dotkotch, R. M. Smith, J. A. SaenzRenauld, H. K. Schnoes, A. L. Burlingame, and D. H. Smith, J. Org. Chem.,34, 3858 (1969).
D. Lavie, E. Glotter, and Y. Shvo, J. Org. Chem.,30, 1774 (1965).
D. Lavie, E. Glotter, and Y. Shvo, J. Chem. Soc., 7517 (1965).
R. N. Tusunova, V. A. Maslennikova, and N. K. Abubakirov, Khim. Prir. soedin., 670 (1976).
D. Lavie, I. Kirson, E. Glotter, D. Rabanovich, and Z. Shakked, J. Chem. Soc. Chem. Commun., 877 (1972).
H. Eggert, C. L. Van Antwerp, N. S. Bhacca, and C. Djerassi, J. Org. Chem.,41, 71, 4051 (1976).
H. Eggert and C. Djerassi, J. Org. Chem.,38, 3788 (1973).
C. Weygand and C. Hilgetag, Organisch-chemische Experimentierkunst, J. A. Barth, Leipzig (1964).
Additional information
Institute of the Chemistry of Plant Substances, Academy of Sciences of the Uzbek SSR, Tashkent. Translated from Khimiya Prirodnykh Soedinenii, No. 2, pp. 197–207, March–April, 1984.
Rights and permissions
About this article
Cite this article
Abdullaev, N.D., Maslennikova, E.A., Tursunova, R.N. et al. Withasteroids ofPhysalis. IV. 28-Hydrowithaphysanolide.13C NMR spectrum of 14-α-hydroxywithasteroids. Chem Nat Compd 20, 182–191 (1984). https://doi.org/10.1007/BF00579480
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00579480