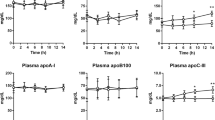
Abstract
The mechanism of triglyceride lowering by Acipimox, a nicotinic acid analogue, was examined in a group of five moderately hypertriglyceridemic male rhesus monkeys. Two experiments were designed to examine the effect of the drug on lipid and glucose metabolism in nondiabetic, insulin-resistant animals. A single dose of Acipimox (8 mg/kg) given with a meal lowered the plasma free fatty acids (FFA) significantly at 4 h (0.102±0.008 vs 0.154±0.020 g/l;\(\bar x\)±SEM;P<0.03); however, FFA concentrations returned to control levels at 6 h. Chronic administration of Acipimox (16 mg/kg q. i. d.) for 2 months produced a 31% reduction in triglyceride concentration (P<0.05) and a significant decrease in low density lipoprotein (LDL)-cholesterol (P<0.04), without changes in insulin action as measured by the hyperinsulinemic euglycemic clamp. Fasting FFA concentrations were not significantly altered by chronic treatment (0.163±0.013 versus 0.140±0.034 g/l). Fatty acid metabolic studies indicated increases in FFA transport (203.7±59.1 versus 136.1±26.6 μEq/min;P<0.05), while FFA fractional clearance rate (FCR) was unchanged. Very low density lipoprotein triglyceride (VLDL-Tg) metabolic experiments, using [3H]glycerol, showed increases in production and FCR with the drug. Increased VLDL-Tg clearance, in spite of increased production of VLDL, appears to be the mechanism by which triglycerides are lowered upon chronic Acipimox administration.
Similar content being viewed by others
References
Ambrogi V, Cozzi P, Sanjust P, Bertone L, Lovisolo PP, Briatico-Vangosa G, Angelucci R, Antilipolytic activity of a series of pyrazine-N-oxides. Eur J Med Chem 15:157–163, 1980
Lovisolo PP, Briatico-Vangosa G, Orsini G, Ronchi R, Angelucci R, Valzelli G, Pharmacological profile of a new anti-lipolytic agent: 5-methyl-pyrazine-2-carboxylic acid 4-oxide (Acipimox) 1. Mechanism of action. Pharm Res Commun 13: 151–162, 1981
Sirtori CR, Gianfranceschi G, Sirtori M, Berini F, Descovich G, Montaguti U, Fuccella LM, Musatti L, Reduced triglyceridemia and increased high density lipoprotein cholesterol levels after treatment with Acipimox, a new inhibitor of lipolysis. Atherosclerosis 38:267–271, 1981
Stirling C, McAleer M, Reckless JPD, Campbell RR, Mundy D, Betteridge DJ, Foster K, Effects of Acipimox, a nicotinic acid derivative, on lipolysis in human adipose tissue and on cholesterol synthesis in human jejunal mucosa. Clin Sci 68: 83–89, 1985
National Cholesterol Education Program Expert Panel. Report on detection, evaluation and treatment of high blood cholesterol in adults. Arch Intern Med 148:36–39, 1988
Canner PL, Berge KG, Wenger NK, Stampler J, Friedman L, Prineas RJ, Friedewald W, Fifteen year mortality in coronary drug project patients: long-term benefit with niacin. J Am Coll Cardiol 8:1245–1255, 1988
Tornvall P, Walldius G, A comparison between nicotinic acid and acipimox on hypertriglyceridemia-effects on serum lipids, lipoproteins, glucose tolerance and tolerability. J Intern Med 230:415–421, 1991
Fuccella LM, Goldaniga G, Lovisolo P, Maggi E, Musatti L, Mandelli V, Sirtori CR, Inhibition of lipolysis by nicotinic acid and by Acipimox. Clin Pharm Ther 28:790–795, 1980
Grundy SM, Mol HYI, Zech L, Berman M, Influence of nicotinic acid on metabolism of cholesterol and triglycerides in man. J Lipid Res 22:24–36, 1981
Hannah JS, Bodkin NB, Hansen BC, Le N-A, Howard BV, Changes in lipoprotein concentrations during the development of noninsulin dependent diabetes mellitus in obese rhesus monkeys. J Clin Endocrinol Metabol 72:1067–1072, 1991
Hansen BC, Bodkin NL, Heterogeneity of insulin responses: phases in the continuum leading to non-insulin-dependent diabetes mellitus. Diabetologia 29:713–719, 1986
Hansen BC, Prospective study of the development of diabetes in spontaneously obese monkeys. In: Berry EM, Blondheim SH, Eliahou HE, Shafir E (eds) Recent advances in obesity research, V. John Libbey, London, pp 33–41, 1987
Bodkin NL, Metzger BL, Hansen BC, Sequential changes in hepatic glucose production and insulin sensitivity during the development of noninsulin-dependent diabetes mellitus in monkeys. Am J Physiol 256:E676-E681, 1989
Kemnitz JW, Kraemer GW, Assessment of glucoregulation in rhesus monkeys sedated with ketamine. Am J Primatol 3: 201–210, 1982
Brady AG, Kornitnik DR, The effects of ketamine anesthesis on glucose clearance in African green monkeys. J Med Primatol 14:99–107, 1982
Taskinen M-R, Bogardus C, Kennedy A, Howard BV, Multiple disturbances of free fatty acid metabolism in noninsulin-dependent diabetes. Effect of oral hypoglycemic therapy. J Clin Invest 76:637–644, 1985
Zweems J, Frankena H, An improved method for the determination of the plasma volume with Evans Blue. J Clin Chem Clin Biochem 19:919–924, 1981
Moore FD, Determination of total body water and solids with isotopes. Science 104:157–160, 1981
Pace N, Kline L, Schachman HK, Harfenist M, Studies on body composition. IV. Use of radioactive hydrogen for measurement in vivo of total body water. J Biol Chem 168:459–469, 1947
Howard BV, Reitman JS, Vasquez B, Zech L, Very-low-density lipoprotein triglyceride metabolism in non-insulin-dependent diabetes mellitus. Diabetes 32:271–276, 1983
Goldberg IJ, Le N-A, Ginsberg HN, Paterniti JR Jr, Brown WV, Metabolism of apoprotein B in cynomolgus monkey: evidence for independent production of low-density lipoprotein apoprotein B. Am J Physiol 244:E196-E201, 1983
Catheart S, Dominiczak MH, The measurement of lipoprotein subfractions in plasma using a tabletop ultracentrifuge. Ann Clin Biochem 27:459–464, 1990
Morgan CR, Lazarow A, Immunology of insulin. Two antibody system-plasma insulin levels of normal, subdiabetic and diabetic rats. Diabetes 12:115–126, 1963
Lipid Research Clinic Program. Manual of Laboratory Operations, 2nd edn. (Publication No. 75-628) US Department of Health, Education, and Welfare, Washington DC, 1982
Allain CC, Poon LS, Chan CSG, Richmond W, Fu PC, Enzymatic determination of total serum cholesterol. Clin Chem 20:470–475, 1974
Wahlefeld A, Triglycerides: determination after enzymatic hydrolysis, 2nd English edn. Academic Press, New York, pp 1831–1835, 1974
Bachorik PS, Albers JJ, Precipitation methods for quantification of lipoproteins. Methods Enzymol 129:78–100, 1986
Zech LA, Boston RC, Foster DM, The methodology of compartmental modeling as applied to the investigation of lipoprotein metabolism. Methods Enzymol 129:366–384, 1986
Saloranta C, Taskinen M-R, Widen E, Harkonen M, Melander A, Groop L, Metabolic consequences of sustained suppression of free fatty acids by Acipimox in patients with NIDDM. Diabetes 42:1559–1566, 1993
Saloranta C, Groop L, Ekstrand A, Franssila-Kallunki A, Taskinen M-R, The effect of an antilipolytic agent (acipimox) on the insulin resistance of lipid and glucose metabolism in hypertriglyceridaemic patients. Acta Diabetol 31:6–13, 1994
Franceschini G, Bernini F, Michelagnoli S, Bellosta S, Vaccarino V, Fumagalli R, Sirtoli CR, Lipoprotein changes and increased affinity of LDL for their receptors after Acipimox treatment in hypertriglyceridemia. Atherosclerosis 81:41–49, 1990
Eisenberg S, Gavish D, Oschry Y, Fainaru M, Deckelbaum RJ, Abnormalities in very low, low, and high density lipoproteins in hypertriglyceridemia: reversal toward normal with Bezafibrate treatment. J Clin Invest 74:470–482, 1984
Saku K, Gartside PS, Hynd BA, Kashyap ML, Mechanism of action of Gemfibrozil on lipoprotein metabolism. J Clin Invest 75:1702–1712, 1985
Garg A, Grundy SM, Nicotinic acid as therapy for dyslipidemia in non-insulin-dependent diabetes mellitus. JAMA 264: 723–726, 1990
Vaag A, Skott P, Damsbo P, Gall M-A, Richer EA, Beck-Nielsen H, Effect of the antilipolytic nicotinic acid analogue Acipimox on whole-body and skeletal muscle glucose metabolism in patients with non-insulin-dependent diabetes mellitus. J Clin Invest 88:1282–1290, 1991
Fulcher GR, Walker M, Catalano C, Agius L, Alberti KGMM, Metabolic effects of suppression of nonesterified fatty acid levels with Acipimox in obese NIDDM subjects. Diabetes 41:1400–1408, 1992
Dulbecco A, Albenga C, Borretta G, Vacca G, Milanesi G, Lavezzari M, Effect of Acipimox on plasma glucose levels in patients with non-insulin-dependent diabetes mellitus. Curr Ther Res 46:478–483, 1989
Lavezzari M, Milanesi G, Oggioni E, Pamparana F, Results of a phase IV study carried out with Acipimox in type II diabetic patients with concomitant hyperlipoproteinemia. J Int Med Res 17:373–380, 1989
Dean JD, McCarthy S, Betteridge DJ, Whately-Smith C, Powell J, Owens DR, The effect of Acipimox in patients with type 2 diabetes and persistent hyperlipidemia. Diabetes Med 9:611–615, 1992
Scott RS, Lintott CJ, Bremer JM, Shand B, Sutherland WHF, Improvement in atherogenic risk factors with Acipimox in noninsulin-dependent diabetic subjects. Atherosclerosis Rev 22:201–206, 1991
Fulcher GR, Catalano C, Walker M, Farrer M, Thow J, Whately-Smith C, Alberti KGMM, A double blind study of the effect of Acipimox on serum lipids, blood glucose control and insulin action in non-obese patients with type 2 diabetes mellitus. Diabetes Med 9:908–914, 1992
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hannah, J.S., Bodkin, N.L., Paidi, M.S. et al. Effects of Acipimox on the metabolism of free fatty acids and very low density lipoprotein triglyceride. Acta Diabetol 32, 279–283 (1995). https://doi.org/10.1007/BF00576264
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00576264